NIAAA Director's Report on Institute Activities to the 154th Meeting of the National Advisory Council on Alcohol Abuse and Alcoholism
Virtual Meeting
Table of Contents
- NIAAA BUDGET
- DIRECTOR'S ACTIVITIES
- STAFF TRANSITIONS
- NATIONAL ADVISORY COUNCIL ON ALCOHOL ABUSE AND ALCOHOLISM
- RECENTLY ISSUED NOTICE OF FUNDING OPPORTUNITIES
- NOTABLE NIAAA STAFF ACTIVITIES
- NIH RESEARCH HIGHLIGHTS
- NIAAA COMMUNICATIONS ACTIVITIES
Fiscal Year (FY) 2020
NIAAA closed FY 2019 on September 30, 2019; the final FY 2019 appropriation for NIAAA was $525.6 million. This represented a $16.0 million (3.1 percent) increase over the FY 2018 budget level. The NIAAA appropriation included a $5.8 million set-aside for the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) initiative. Key funding actions within this appropriation were:On December 20, 2019, the President signed H.R.1865 - Further Consolidated Appropriations Act, 2020 Public Law No. 116-94. NIH received a total of $41.6 billion, $2.3 billion above the FY 2019 enacted level. This funding includes allocations for the Helping to End Addiction Long-term (HEAL) Initiative, the 21st Century Cures Act, the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative, and research on influenza. The bill provides a general increase to NIH institutes and Centers and it continues support for the Gabriella Miller Kids First Act pediatric research initiative.
In addition to the FY 2020 appropriation, NIH received $836 million in supplemental funds to respond to the coronavirus pandemic (H.R.6074 - Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020, Pub. L. 116-123) enacted March 6, 2020. On March 27, 2020, President Trump signed the Coronavirus Aid, Relief, and Economic Security (CARES) Act, Pub. L. 116-136, into law, which provided NIH with an additional $945.5M for vaccine, therapeutic, and diagnostic research including underlying risks to cardiovascular and pulmonary conditions.
The FY 2020 appropriation for NIAAA provides $545.4 million. This represents a $19.8 million (3.8 percent) increase over the FY 2019 budget level. NIAAA estimates it will support a total of 778 research project grants (RPGs) in FY 2020.
FY 2021
The FY 2021 budget is under development.
NIAAA Director Dr. George F. Koob gave the following presentations between January 1, 2020, and April 30, 2020:
- “NIAAA at 50: Providing Information and Tools for Communities to Address Alcohol Problems” for the Community Anti-Drug Coalitions of America in National Harbor, Maryland, on February 4, 2020
- “50 Years of Neuroscience Research at NIAAA: From Membranes to Microcircuits (Allosterism, Hyperkatifiea, and Allostasis)” for the Alcohol and the Nervous System Gordon Research Conference in Galveston, Texas, on March 1, 2020
- “The View from the National Institutes of Health: NIAAA Update on Advances in the Prevention, Diagnosis, and Treatment of Alcohol Use Disorder” for the American Psychopathological Association in New York, New York, on March 5, 2020
- “Big Ideas: How Digital Health is Shaping the Future of Addiction Medicine” for the American Society of Addiction Medicine Virtual Meeting on April 3, 2020 • “Bridging the Gap Between Science and Practice: NIAAA Perspective” for the American Society of Addiction Medicine Virtual Meeting on April 14, 2020
- “NIAAA 50th Anniversary, Alcohol Use Disorder: The Brain, the Pain and Alleviating the Shame” for the American Psychiatric Association Virtual Meeting on April 26, 2020
New Staff

Dr. Elma Aflaki joined the Laboratory of Molecular Signaling (LMS) in the Division of Intramural Clinical and Biological Research as a Staff Scientist. Before joining NIAAA, Dr. Aflaki completed her Ph.D. in 2011 at Medical University of Graz, Austria, where she studied the role of adipose triglyceride lipase in macrophage inflammation, cytoskeleton rearrangement, and cell survival. She subsequently joined the National Human Genome Research Institute as a Visiting Fellow to investigate Parkinson’s disease and Gaucher disease using human induced pluripotent stem cells (iPSCs). In LMS, Dr. Aflaki will design and perform biochemical, molecular and cell biological studies in iPSC-derived neural cells. Dr. Aflaki will also examine the implication of GPR110 in central nervous system injury outcome and neurodegenerative conditions including ethanol exposure in cell and animal models.

Dr. Michelle Antoine joined the Division of Intramural Clinical and Biological Research as an NIH Earl Stadtman Tenure-Track investigator and 2019 NIH Distinguished Scholar. She will lead the Section on Neural Circuits in the NIAAA Office of the Scientific Director. Dr. Antoine’s research focuses on deciphering the genetic and environmental factors that impair neurocircuit activity and contribute to neurodevelopmental disorders such as fetal alcohol spectrum disorders, autism spectrum disorders, and attention deficit hyperactivity disorder. Dr. Antoine earned her B.S. in Biology and Mathematics from Spelman College in 2004 and her M.S. and Ph.D. from the Albert Einstein College of Medicine in 2013. She completed postdoctoral training as a Miller Research Fellow in the Helen Wills Neuroscience Institute at the University of California, Berkeley.

Dr. Salma Majid was appointed Staff Scientist in the Laboratory of Neurogenetics (LNG), Division of Intramural Clinical and Biological Research. Dr. Majid received her Ph.D. in Toxicology in 2002 from Hamdard University National Institute of Immunology in New Delhi, India. In LNG, Dr. Majid is spearheading an initiative to evaluate sequence variants for functional significance by gene editing human cell lines, including induced pluripotent stem cells.

Commander (CDR) LaToya Sewell of the United States Public Health Service joined the Office of the Clinical Director in the Division of Intramural Clinical and Biological Research. CDR Sewell is a board-certified Family Nurse Practitioner with more than 14 years of experience. CDR Sewell earned her bachelor’s degree from Towson University in 2002, completed the National Institutes of Health Neuroscience Nurse Internship Program in 2003, and earned her master’s degree from the University of Maryland at Baltimore in 2006. She has previously worked in the fields of detention health, primary and urgent care, alcohol use disorder, autonomic function disorders, and neuro-oncology..
Internal Transitions

Dr. Raouf Kechrid has been appointed Facility Head of the Office of Laboratory Animal Science (OLAS) in the Division of Intramural Clinical and Biological Research. OLAS oversees the animal care and use program of the NIAAA intramural research program and ensures compliance with the Institute for Laboratory Animal Research Guide.

Dr. Peter Menza has been converted from a Post-Doctoral Intramural Research Training Award (IRTA) to a Research Fellow in the Laboratory of Neuroimaging, Division of Intramural Clinical and Biological Research. Dr. Menza will coordinate various aspects of imaging clinical trials, develop and administer neuropsychological evaluations, and establish new imaging paradigms and tools for analysis of imaging and other data.

Dr. Vijay Ramchandani has been promoted to tenured Senior Investigator. Dr. Ramchandani is Chief of the Section on Human Psychopharmacology in the Division of Intramural Clinical and Biological Research. Using intravenous alcohol administration and pharmacokinetic modeling, his research focuses on identifying risk factors and developing treatments for alcohol use disorder (AUD). His research spans genetic, environmental, and neurobiological factors that affect alcohol response, advancing understanding of how variability in alcohol response influences the risk of developing AUD.
Departing Staff
Gabriela Coello, formerly of NIAAA’s Administrative Services Branch, accepted a new position at the National Cancer Institute where she will now serve as the Administrative Officer for the Surgery Branch.
NATIONAL ADVISORY COUNCIL ON ALCOHOL ABUSE AND ALCOHOLISM

New council members were recently appointed to serve on the National Advisory Council on Alcohol Abuse and Alcoholism.
Back row, from left: Dr. Christopher Carpenter, Dr. Laura Nagy, Dr. Laura O’Dell, and Dr. Charles H. Lang
Front row, from left: Dr. Patricia Powell (NIAAA Deputy Director), Ms. Beth Kane-Davidson, Dr. George F. Koob (NIAAA Director), and Dr. Mary Larimer
RECENTLY ISSUED NOTICE OF FUNDING OPPORTUNITIES
Notice of Funding Opportunities (NOFO) Issued by NIAAA:
Consortium on the Neurobiology of Adolescent Drinking in Adulthood (U01 - Clinical Trial Not Allowed; U24 Clinical Trial Not Allowed): This NOFO supports research projects and research resource cooperative agreements as components of the Neurobiology of Adolescent Drinking in Adulthood (NADIA) consortium to elucidate persistent changes in complex brain function-behavior relationships following adolescent alcohol exposure. RFA-AA-20-003; RFA AA 20-004; RFA AA-20-005
Impact of Alcohol on the Onset and Progression of Alzheimer’s Disease and Its Related Dementias (R01 - Clinical Trial Optional): In collaboration with National Institute on Aging, this NOFO supports basic and clinical research on the influence of alcohol on susceptibility and progression of Alzheimer's disease and its related dementias. RFA-AA-20-006
Medications Development for the Treatment of Alcohol Use Disorder (AUD) or Alcohol-Related Organ Damage (AROD), or the Combination of AUD and AROD (U01 - Clinical Trial Optional): The purpose of this NOFO is to support cooperative agreement applications for research that advances promising compounds through the drug development pipeline for the treatment of alcohol use disorder (AUD) or alcohol-related organ damage (AROD), or the combination of AUD and AROD; and move candidate compounds through any single phase, or multiple phases of the development spectrum. RFA-AA-20-007
Notices of Special Interest (NOSIs) Issued by NIAAA:
Availability of Administrative Supplements and Competitive Revision Supplements on Coronavirus Disease 2019 (COVID-19) within the Mission of NIAAA: This notice solicits science-focused supplements in a number of research areas that are prioritized by NIAAA as listed in the NOSI, including those that capitalize on existing research cohorts, to investigate urgent research questions of significance to the COVID-19 pandemic within the general population and among underserved populations, such as racial, ethnic, and gender minorities, individuals with low socioeconomic status, and those who are incarcerated or homeless. NOT-AA-20-011
NIH-Wide NOFOs with NIAAA Participation:
Research on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) (R01 - Clinical Trials Not Allowed; R21 Clinical Trials Not Allowed) PAR-20-165; PAR-20-168
Limited Competition: International Epidemiology Databases to Evaluate AIDS (IeDEA) (U01 - Clinical Trial Not Allowed) RFA-AI-20-023
RAIN Initiative: Exploratory Team-Research BRAIN Circuit Programs - eTeamBCP (U01 - Clinical Trials Optional) RFA-NS-20-029
Mechanisms and Consequences of Sleep Disparities in the U.S. (R01 - Clinical Trial Not Allowed) PAR-20-164
Firearm Injury and Mortality Prevention Research (R61 - Clinical Trial Optional) PAR-20-143
Emergency Competitive Revision to Existing NIH Awards (Emergency Supplement - Clinical Trial Optional) PA-20-135
Blueprint Neurotherapeutics Network (BPN): Small Molecule Drug Discovery and Development for Disorders of the Nervous System (U44 - Clinical Trial Optional; UG3/UH3 - Clinical Trial Optional) PAR-20-111; PAR-20-122
National Cooperative Drug/Device Discovery/Development Groups (NCDDG) for the Treatment of Mental or Substance Use Disorders or Alcohol Disorder (U19 - Clinical Trial Optional) PAR-20-119
BRAIN Initiative: Standards to Define Experiments Related to the BRAIN Initiative (R01 - Clinical Trial Not Allowed) RFA-MH-20-128
HEAL Initiative: Pain Management Effectiveness Research Network: Clinical Trial Planning and Implementation Cooperative Agreement (UG3/UH3 - Clinical Trial Required) RFA-NS-20-028
Biomedical Knowledgebase (U24 - Clinical Trials Not Allowed) PAR-20-097
Biomedical Data Repository (U24 - Clinical Trials Not Allowed) PAR-20-089
NIH-Wide NOSIs with NIAAA participation:
Availability of Administrative Supplements and Urgent Competitive Revisions for Research on the 2019 Novel Coronavirus and the Behavioral and Social Sciences NOT-AG-20-097
Availability of Administrative Supplements and Urgent Competitive Revisions for Research on Stress Management in Relation to Coronavirus Disease 2019 (COVID-19) NOT-AT-20-011
Availability of Administrative Supplements and Urgent Competitive Revisions for Mental Health Research on the 2019 Novel Coronavirus NOT-MH-20-047
HEAL Initiative: Back Pain Consortium (BACPAC) Research Program Notice of Special Interest (NOSI) regarding the Availability of Urgent Competitive Revisions NOT-AR-20-012; NOT-AR-20-016
HEAL Supplements to Improve the Treatment and Management of Common Co-occurring Conditions and Suicide Risk in People Affected by the Opioid Crisis NOT-MH-20-025
Ancillary Reproductive Health Projects to Existing Large and/or Longitudinal Studies NOT-HD-20-008
Health Services Research on Minority Health and Health Disparities (R01 - Clinical Trial Optional) NOT-MD-20-011
Research to Improve the Interpretation of Patient-Reported Outcomes at the Individual Patient Level for Use in Clinical Practice NOT-OD-20-079
Competitive Revisions for Firearms Injury and Mortality Prevention Research NOT-OD-20-089
Notice of Special Interest to Encourage Eligible NIH HEAL Initiative Awardees to Apply for PA-18-906 Research Supplements to Promote Diversity in Health-Related Research (Admin Supp - Clinical Trial Not Allowed) NOT-NS-20-023
Administrative Supplements to Support Enhancement of Software Tools for Open Science NOT-OD-20-073
Administrative Supplements for Research on Women’s Health in the IDeA States NOT-GM-20-017
Administrative Supplement for Continuity of Biomedical and Behavioral Research Among First-Time Recipients of NIH Research Project Grant Awards NOT-OD-20-055
NOTABLE NIAAA STAFF ACTIVITIES
Dr. Ralph Hingson presented “Trends and Interventions that Work to Prevent Underage Drinking” and Dr. Aaron White presented “Alcohol and Opioids – A Deadly Combination” for the Community Anti-Drug Coalitions of America National Leadership Forum on February 4, 2020, in National Harbor, Maryland.
Dr. Raye Litten presented “Medications with Abuse Potential for Treatment of Alcohol Use Disorder” at the International Society of CNS Clinical Trials and Methodology (ISCTM) in Washington, D.C., on February 19, 2020.
Dr. Mariela Shirley was a member of the NIH and Food and Drug Administration (FDA) planning committee that organized a two-day meeting, “E-Cigarette Prevention and Cessation in Youth and Young Adults”, on March 2 and 3, 2020. NIH’s National Heart, Lung, and Blood Institute and Office of Behavioral and Social Sciences Research served as leads in the planning and coordination of this event.
FETAL ALCOHOL SPECTRUM DISORDER PREDISPOSES TO METABOLIC ABNORMALITIES IN ADULTHOOD
Significance: The potential contribution of prenatal alcohol exposure (PAE) to the etiology of chronic diseases and health conditions later in life is not well understood. In this study, analysis of data from a patient registry found that adult patients with FASD had an increased incidence of type 2 diabetes, lower HDL “good” cholesterol, and elevated triglyceride levels compared than those without FASD. Using a zebrafish model of PAE, researchers examined the biological and molecular connections between these metabolic abnormalities and PAE. They demonstrate that PAE was associated with obesity, fasting hyperglycemia, increased abdominal fat, and abnormal liver development in adult fish challenged with a high-fat, high-cholesterol diet. This study has implications for optimizing strategies for disease prevention in individuals with prenatal alcohol exposure.
Prenatal alcohol exposure (PAE) affects at least 10% of newborns globally and leads to the development of fetal alcohol spectrum disorders (FASDs). Despite its high incidence, there is no consensus on the implications of PAE on metabolic disease risk in adults. Here, we describe a cohort of adults with FASDs that had an increased incidence of metabolic abnormalities, including type 2 diabetes, low HDL, high triglycerides, and female-specific overweight and obesity. Using a zebrafish model for PAE, we performed population studies to elucidate the metabolic disease seen in the clinical cohort. Embryonic alcohol exposure (EAE) in male zebrafish increased the propensity for diet-induced obesity and fasting hyperglycemia in adulthood. We identified several consequences of EAE that may contribute to these phenotypes, including a reduction in adult locomotor activity, alterations in visceral adipose tissue and hepatic development, and persistent diet-responsive transcriptional changes. Taken together, our findings define metabolic vulnerabilities due to EAE and provide evidence that behavioral changes and primary organ dysfunction contribute to resultant metabolic abnormalities. (Weeks O*, Bossé GD, Oderberg IM**, Akle S, Houvras Y, Wrighton PJ**, LaBella K, Iversen I, Tavakoli S, Adatto I, Schwartz A, Kloosterman D, Tsomides A, Charness ME, Peterson RT, Steinhauser ML, Fazeli PK, and Goessling W. J Clin Invest. 2020 Mar 23. pii: 132139. doi: 10.1172/JCI132139.) *NIAAA F31 trainee **NIAAA F32 trainee
KCNN2 BLOCKADE REVERSES LEARNING DEFICITS IN A MOUSE MODEL OF FETAL ALCOHOL SPECTRUM DISORDERS
Significance: Researchers previously found that alcohol-induced activation of heat shock factor 1 leads to specific epigenetic changes in nearly 100 genes that can result in long-term impacts on the neurobehavior of offspring. In the current study, researchers examined one of those changes—the increased expression of a small-conductance calcium-activated potassium channel, Kcnn2, in the motor area of the cerebral cortex of the brain. The research demonstrated that increased Kcnn2 expression correlated with deficits in motor skill learning caused by prenatal alcohol exposure that were mitigated by pharmacological blockade of Kcnn2. This study not only presents an experimental system to identify potential targets for FASD treatment in brain and non-brain tissues, but also provides early evidence for Kcnn2 blockade as a potential pharmacotherapy for FASD-related learning disabilities.
Learning disabilities are hallmarks of congenital conditions caused by prenatal exposure to harmful agents. These include fetal alcohol spectrum disorders (FASDs) with a wide range of cognitive deficiencies, including impaired motor skill development. Although these effects have been well characterized, the molecular effects that bring about these behavioral consequences remain to be determined. We previously found that the acute molecular responses to alcohol in the embryonic brain are stochastic, varying among neural progenitor cells. However, the pathophysiological consequences stemming from these heterogeneous responses remain unknown. Here we show that acute responses to alcohol in progenitor cells altered gene expression in their descendant neurons. Among the altered genes, an increase of the calcium-activated potassium channel Kcnn2 in the motor cortex correlated with motor learning deficits in a mouse model of FASD. Pharmacologic blockade of Kcnn2 improves these learning deficits, suggesting Kcnn2 blockers as a new intervention for learning disabilities in FASD. (Mohammad S, Page SJ, Wang L , Ishii S, Li, P, Sasaki T ,Basha A, Salzberg A, Quezado Z , Imamura F, Nishi H, Isaka K, Corbin JG, Liu JS, Imamura Kawasawa Y, Torii M, and Hashimoto-Torii K. Nat Neurosci. 2020 Mar 16. doi: 10.1038/s41593-020-0592-z.)
CONCURRENT PRENATAL DRINKING AND SMOKING INCREASES RISK FOR SIDS: SAFE PASSAGE STUDY REPORT
Significance: To investigate the association between prenatal alcohol and tobacco exposure on the risk of sudden infant death syndrome (SIDS), a network of researchers conducted a large-scale study of the outcomes of nearly 12,000 pregnancies among women from multiple study sites with high rates of prenatal alcohol use and SIDS. Researchers found that infants prenatally exposed to both alcohol and cigarettes beyond the first trimester have a substantially higher risk for SIDS compared to those unexposed, exposed to alcohol or cigarettes alone, or when the mother reported quitting early in pregnancy. These findings further emphasize the role of the early prenatal environment for healthy postnatal outcomes and suggest that screening for substance use early in pregnancy and intervening as soon as possible may help address this public health concern.
BACKGROUND: Sudden infant death syndrome (SIDS) is the leading cause of postneonatal mortality. Although the rate has plateaued, any unexpected death of an infant is a family tragedy thus finding causes and contributors to risk remains a major public health concern. The primary objective of this investigation was to determine patterns of drinking and smoking during pregnancy that increase risk of SIDS. METHODS: The Safe Passage Study was a prospective, multi-center, observational study with 10,088 women, 11,892 pregnancies, and 12,029 fetuses, followed to 1-year post delivery. Subjects were from two sites in Cape Town, South Africa and five United States sites, including two American Indian Reservations. Group-based trajectory modeling was utilized to categorize patterns of drinking and smoking exposure during pregnancy. FINDINGS: One-year outcome was ascertained in 94·2% infants, with 28 SIDS (2·43/1000) and 38 known causes of death (3·30/1000). The increase in relative risk for SIDS, adjusted for key demographic and clinical characteristics, was 11·79 (98·3% CI: 2·59-53·7, p < 0·001) in infants whose mothers reported both prenatal drinking and smoking beyond the first trimester, 3.95 (98·3% CI: 0·44-35·83, p = 0·14), for drinking only beyond the first trimester and 4·86 (95% CI: 0·97-24·27, p = 0·02) for smoking only beyond the first trimester as compared to those unexposed or reported quitting early in pregnancy. INTERPRETATION: Infants prenatally exposed to both alcohol and cigarettes continuing beyond the first trimester have a substantially higher risk for SIDS compared to those unexposed, exposed to alcohol or cigarettes alone, or when mother reported quitting early in pregnancy. Given that prenatal drinking and smoking are modifiable risk factors, these results address a major global public health problem. (Elliott AJ, Kinney HC, Haynes RL, Dempers JD, Wright C, Fifer WP, Angal J, Boyd TK, Burd L, Burger E, Folkerth RD, Groenewald C, Hankins G, Hereld D, Hoffman HJ, Holm IA, Myers MM, Nelsen LL, Odendaal HJ, Petersen J, Randall BB, Roberts DJ, Robinson F, Schubert P, Sens MA, Sullivan LM, Tripp T, Van Eerden P, Wadee S, Willinger M, Zaharie D, and Dukes KA. E Clinical Medicine. 2020 Jan 20;19:100247. doi: 10.1016/j.eclinm.2019.100247.)
Significance: Immune signaling and mediators, known to be significant in alcohol-induced liver damage, have been recently linked to alcohol drinking-related behavior and neuropathology. Using mouse models, this study demonstrated that the pro-inflammatory cytokine IL-17 is involved in both alcohol-induced liver and brain injury and in voluntary drinking behavior. The study also found that circulating levels of IL-17 are elevated in both humans who drink alcohol excessively and in alcohol-dependent mice. Most significantly, results demonstrated that pharmacological blockade of IL-17 effectively reduces the alcohol-induced liver/brain damage and voluntary drinking in mice, supporting the translational potential of this approach for treatment of alcohol-related pathology in humans.
Chronic alcohol abuse has a detrimental effect on the brain and liver. There is no effective treatment for these patients, and the mechanism underlying alcohol addiction and consequent alcohol-induced damage of the liver/brain axis remains unresolved. We compared experimental models of alcoholic liver disease (ALD) and alcohol dependence in mice and demonstrated that genetic ablation of IL-17 receptor A (IL-17ra-/-) or pharmacological blockade of IL-17 signaling effectively suppressed the increased voluntary alcohol drinking in alcohol-dependent mice and blocked alcohol-induced hepatocellular and neurological damage. The level of circulating IL-17A positively correlated with the alcohol use in excessive drinkers and was further increased in patients with ALD as compared with healthy individuals. Our data suggest that IL-17A is a common mediator of excessive alcohol consumption and alcohol-induced liver/brain injury, and targeting IL-17A may provide a novel strategy for treatment of alcohol-induced pathology. (Xu J et al., Brenner DA, Koob GF, and Kisseleva T. JCI Insight. 2020 Feb 13;5(3). pii: 131277. doi: 10.1172/jci.insight.131277.)
SINGLE CELL TRANSCRIPTOME PROFILING OF THE HUMAN ALCOHOL-DEPENDENT BRAIN
Significance: In this study, investigators applied a powerful high-throughput tool, single-cell RNA sequencing (scRNA-seq), to examine gene expression changes in individual cells of the human prefrontal cortex, a key brain region vulnerable to AUD. They found that chronic alcohol exposure altered the expression of multiple genes, both protein coding and non-coding, in all neuronal cell types identified. The most pronounced expression changes were identified in non-neuronal cells, including astrocytes, oligodendrocytes and microglia. Furthermore, each cell type, both neuronal and non-neuronal, displayed an increase in the expression of genes linked to neuroinflammation, a process previously associated with excessive alcohol use. This is the first study to demonstrate the powerful capability of single-cell sequencing technology to dissect the complex cell types and gene networks altered in the brains of individuals with AUD.
Alcoholism remains a prevalent health concern throughout the world. Previous studies have identified transcriptomic patterns in the brain associated with alcohol dependence in both humans and animal models. But none of these studies have systematically investigated expression within the unique cell types present in the brain. We utilized single nucleus RNA sequencing (snRNA-seq) to examine the transcriptomes of over 16 000 nuclei isolated from prefrontal cortex of alcoholic and control individuals. Each nucleus was assigned to one of seven major cell types by unsupervised clustering. Cell type enrichment patterns varied greatly among neuroinflammatory-related genes, which are known to play roles in alcohol dependence and neurodegeneration. Differential expression analysis identified cell type-specific genes with altered expression in alcoholics. The largest number of differentially expressed genes (DEGs), including both protein-coding and non-coding, were detected in astrocytes, oligodendrocytes, and microglia. To our knowledge, this is the first single cell transcriptome analysis of alcohol-associated gene expression in any species, and the first such analysis in humans for any addictive substance. These findings greatly advance understanding of transcriptomic changes in the brain of alcohol-dependent individuals. (Brenner E, Tiwari GR, Kapoor M, Liu Y, Brock A, and Mayfield RD. Hum Mol Genet. 2020;ddaa038. doi:10.1093/hmg/ddaa038.)
Significance: Fatty acid amide hydrolase (FAAH), an enzyme that metabolizes the endogenous cannabinoid anandamide, has been implicated in preclinical research as playing a role in alcohol use disorder (AUD). Positron emission tomography of the brain was used with a carbon-11 radiotracer to measure FAAH binding in individuals with AUD during both early and protracted abstinence from alcohol compared to healthy controls. Brain levels of FAAH were lower during early abstinence (3–7 days after cessation of alcohol consumption) in individuals with AUD and were correlated with a higher number of drinks per week prior to abstinence and elevated anandamide levels. FAAH levels, however, appeared to normalize after 2–4 weeks of monitored abstinence. These findings suggest that an endocannabinoid-elevating process may be associated with heavy drinking in AUD, and further suggest a unique target for therapeutic intervention in the brain.
The endocannabinoid enzyme, fatty acid amide hydrolase (FAAH), has been proposed as a therapeutic target for alcohol use disorder (AUD) and co-morbid psychiatric illnesses. Investigating this target in the living human brain and its relationship to clinical outcome is a critical step of informed drug development. Our objective was to establish whether brain FAAH levels are low in individuals with AUD and related to drinking behavior. In this pilot study, treatment-seeking patients with AUD completed two PET scans with the FAAH radiotracer [C-11]CURB after 3-7 days (n = 14) and 2-4 weeks (n = 9) of monitored abstinence. Healthy controls (n = 25) completed one scan. FAAH genetic polymorphism (rs324420) and blood concentrations of anandamide and other N-acylethanolamines metabolized by FAAH were determined and AUD symptoms assessed. In AUD, brain FAAH levels were globally lower than controls during early abstinence (F(1,36) = 5.447; p = 0.025)) and FAAH substrates (anandamide, oleoylethanolamide, and N-docosahexaenoylethanolamide) were significantly elevated (30-67%). No significant differences in FAAH or FAAH substrates were noted after 2-4 weeks abstinence. FAAH levels negatively correlated with drinks per week (r = -0.57, p = 0.032) and plasma concentrations of the three FAAH substrates (r > 0.57; p < 0.04)). Our findings suggest that early abstinence from alcohol in AUD is associated with transiently low brain FAAH levels, which are inversely related to heavier alcohol use and elevated plasma levels of FAAH substrates. Whether low FAAH is an adaptive beneficial response to chronic alcohol is unknown. Therapeutic strategies focusing on FAAH inhibition should consider the possibility that low FAAH during early abstinence may be related to drinking. (Best LM, Williams B, Le Foll B, Mansouri E, Bazinet RP, Lin L, De Luca V, Lagzdins D, Rusjan P, Tyndale RF, Wilson AA, Hendershot CS, Heilig M, Houle S, Tong J, Kish SJ, and Boileau I. Neuropsychopharmacology. 2020 Jan 7. doi: 10.1038/s41386-020-0606-2.)
Significance: To determine whether past suicidality affects the course of alcohol use disorder (AUD), researchers examined whether previous suicidal ideation or attempts are associated with treatment response in individuals with AUD. Participants undergoing detoxification and residential treatment for AUD were assessed for history of suicidal ideation with or without suicide attempts, and alcohol craving was measured weekly during treatment. Individuals with a history of suicide attempts showed higher levels of craving throughout treatment compared to those without a history of suicidality. These results support current guidelines on assessing suicidal ideation in patients with substance use disorders.
BACKGROUND: Alcohol use is associated with an increased risk of completed suicide, but it is unclear whether past suicidality affects the course of alcohol use disorder (AUD). We examined whether a history of suicidal ideation or attempts is associated with treatment response in individuals with AUD. METHODS: 146 participants underwent inpatient detoxification and residential treatment for AUD. Reductions in craving during treatment were used as an index of treatment response. Participants were assessed for history of suicidality using the Columbia-Suicide Severity Rating Scale and divided into three groups: no history of suicidal ideation or attempts (N = 76), history of suicidal ideation without attempts (N = 50), and history of suicide attempts (N = 20). Alcohol craving was measured weekly during treatment using the Penn Alcohol Craving Scale and compared across groups. RESULTS: Individuals with a history of suicide attempts showed higher levels of craving throughout treatment compared to those without a history of suicidality. Associations between past suicide attempts and craving remained significant after adjusting for age, sex, alcohol use disorder severity, comorbid psychopathology, and benzodiazepine treatment. Participants in all groups had significant reductions in alcohol craving by the end of treatment. CONCLUSIONS: Our findings suggest that a history of suicide attempts is associated with higher levels of craving throughout inpatient treatment for AUD. These results support current guidelines on assessing suicidal ideation in patients with substance use disorders. (Janakiraman R, Gowin JL, Sloan ME, Schwandt ML, Diazgranados N, Ramchandani VA, and Kwako LE. Drug Alcohol Depend. 2020 Apr 1;209:107918. doi: 10.1016/j.drugalcdep.2020.107918.)
ALCOHOLICS ANONYMOUS AND OTHER 12-STEP PROGRAMS FOR ALCOHOL USE DISORDER
Significance: Alcoholics Anonymous (AA) has been a common recovery resource for people with alcohol use disorder (AUD) for over 80 years, but only recently has rigorous research on its effectiveness been conducted. This systematic review examined outcomes of over 10,000 participants from 27 studies that compared peer-led AA or professionally delivered Twelve-Step Facilitation (TSF) with other behavioral interventions, such as motivational enhancement therapy or cognitive-behavioral therapy, TSF treatment variants, or no treatment. Across a variety of measures—including the length of time participants abstained from alcohol, reduction in drinking intensity, and health care costs—AA performed at least as well as other behavioral treatments for AUD, and AA was more effective in increasing abstinence. These results suggest that AA and TSF can offer a low-cost, effective treatment option for maintaining abstinence among those with AUD.
Background. Alcohol use disorder (AUD) confers a prodigious burden of disease, disability, and premature mortality, and confers high economic costs from lost productivity, accidents, violence, incarceration, and increased health care utilization. For over 80 years, Alcoholics Anonymous (AA) has been a ubiquitous AUD recovery resource with millions of members, free at the point of access, but it is only more recently that rigorous research on its effectiveness has been conducted. Objectives. To evaluate whether peer-led AA and professionally-delivered treatments that facilitate AA involvement (Twelve-Step Facilitation [TSF] interventions) achieve important outcomes: abstinence (proportion of patients completely abstinent; percent days abstinent [PDA]; longest period of abstinence); reduced drinking intensity (drinks per drinking day [DDD]; percent days heavy drinking [PDHD]); and reduced alcohol-related consequences and alcohol addiction severity. Health care cost-offsets from AA/TSF participation were also evaluated. Search methods. We searched the Cochrane Drugs and Alcohol Group Specialised Register (via CRSLive), Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Embase, CINAHL and PsycINFO from inception to 2 August 2019. We also searched for ongoing and unpublished studies via ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) . All searches included non-English language literature. We hand searched references of topic-related systematic reviews and the included studies. Selection criteria. Randomized controlled trials (RCTs), quasi-RCTs and non-randomized studies that compared AA/TSF with other interventions such as motivational enhancement therapy (MET) or cognitive-behavioral therapy (CBT), TSF treatment variants, or no treatment. Health care cost-offset studies were also included. Participants were non-coerced male and female adults with AUD. Data collection and analysis. We followed the standard methodological procedures established by Cochrane including conducting meta-analyses where appropriate. Studies were categorized by study design (RCT/quasi-RCT; non-randomized; economic); degree of standardized manualization (all interventions manualized vs some/none); and comparison intervention type (i.e., whether AA/TSF was compared to a different theoretical orientation or an AA/TSF intervention that varied in style or intensity). Authors' conclusions. For Proportion of Patients Completely Abstinent, manualized AA/TSF interventions were somewhat more effective than other well-established treatments. Non-manualized AA/TSF may perform as well as other well-established treatments. For other alcohol-related outcomes ,both manualized and non-manualized AA/TSF interventions may be at least as effective as other well-established treatments. Implementing AA/TSF will probably produce substantial health care cost savings among patients with alcohol use disorder. (Kelly J, Humphreys K, and Ferri M. Cochrane Database Syst Rev 3(3) CD012880 2020 Mar 11. doi: 10.1002/14651858.CD012880.pub2.)
NIAAA COMMUNICATIONS ACTIVITIES
Press and Publications Activities
Dr. Koob continues to speak with a variety of national and international news outlets on timely topics related to NIAAA’s research and its impact on treatment and prevention of alcohol misuse and AUD. Notably, the ongoing COVID-19 pandemic has led to multiple media inquiries regarding the pandemic’s impact on alcohol use, the availability of alcohol treatment, and the effects of alcohol use on immune function. Interviews by Dr. Koob and other staff since January 2020 include The New York Times, NPR, NBC, Associated Press, Scripps TV, U.S. News & World Report, Newsweek, Huffington Post, Well and Good, Business Insider, CQ Researcher, Bloomberg News, The Arizona Republic, and numerous other outlets.
Press Releases:
• National Drug and Alcohol Facts Week® celebrates 10 years (March 23, 2020)
• Dr. Laura E. Nagy to Deliver 24th Annual Mark Keller Honorary Lecture at the National Institutes of Health (January 22, 2020)
Publication Statistics: In the month of February 2020, NIAAA filled orders for 8,216 copies of print publications. As of March 27, 2020, there were 27,880 Granicus/GovDelivery listserv subscribers to Alcohol Research: Current Reviews; 23,701 to the NIAAA Spectrum; 1,220 to News Alerts; and 22,563 to receive general information.
New Publications:
• Recuerdos interrumpidos: lagunas mentales inducidas por el alcohol (Spanish translation of Interrupted Memories: Alcohol-Induced Blackouts)
• Las mujeres y el alcohol (Spanish translation of Women and Alcohol)
• Resaca (Spanish translation of Hangovers)
Social Media Highlights
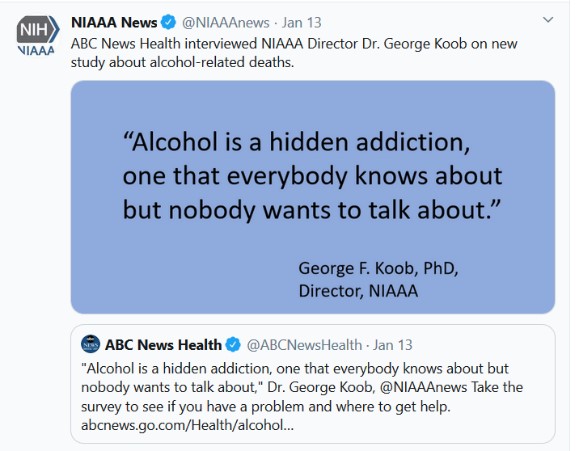 NIAAA Twitter: NIAAA’s Twitter account (@NIAAAnews) currently has more than 25,000 followers and averages about 182,000 impressions per month. Posts focused on treatment options, Alcohol Awareness Month, and interviews by Dr. Koob, such as an ABC News Health tweet on alcohol being considered a “hidden addiction.”
NIAAA Twitter: NIAAA’s Twitter account (@NIAAAnews) currently has more than 25,000 followers and averages about 182,000 impressions per month. Posts focused on treatment options, Alcohol Awareness Month, and interviews by Dr. Koob, such as an ABC News Health tweet on alcohol being considered a “hidden addiction.”
Recently, tweets have focused on COVID-19, physical distancing, social isolation, and telehealth resources.
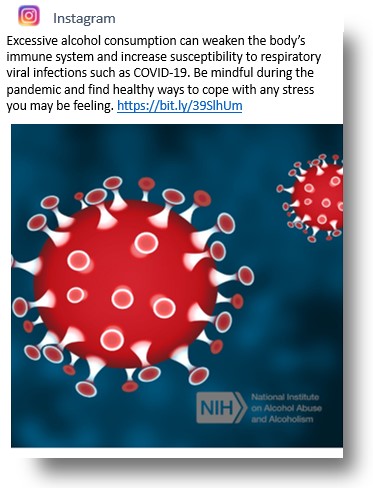
Instagram: NIAAA’s Instagram account (@NIAAAnews) now has more than 1,000 followers. Recent posts include messages about alcohol treatment options during physical distancing, the NIAAA Alcohol Treatment Navigator, fetal alcohol spectrum disorders, Alcohol Awareness Month, and recognition of International Day of Women and Girls in Science and National Doctor’s Day. Recently, Instagram posts also have focused on the COVID-19 pandemic, physical distancing, and how to find telehealth and virtual meeting support resources for people in recovery.
NIAAA Director’s Blog: In March 2020, Dr. Koob updated his blog with an entry about rethinking our drinking habits and the trend toward taking a break from drinking alcohol.
In April 2020, a new post was added that describes the challenges posed by alcohol during the COVID-19 pandemic.
NIAAA Digital Promotion Campaign for Spanish language publications: NIAAA is promoting Spanish-language publications through posts on Google, Twitter, Facebook, and Instagram.
Exhibits
 NBC4/Telemundo Health & Fitness Expo: On January 18 and 19, 2020, NIAAA exhibited at the NBC4/Telemundo Health and Fitness Expo in Washington, D.C. An estimated 70,000 people attend this annual event. NIAAA had an exhibit booth offering general information about alcohol and health, as well as the NIAAA Treatment Navigator. As part of this year’s booth package, NIAAA was able to benefit from a partnership NBC4 had formed with Quest Diagnostics to show video public service announcements (PSAs) on television screens inside public waiting rooms at Quest facilities. During the two weeks leading up to the Expo, a 30-second PSA promoting the NIAAA Alcohol Treatment Navigator was shown on TV monitors in 47 waiting rooms located in the local D.C. area, with an estimated 150,000 impressions. Watch the PSA.
NBC4/Telemundo Health & Fitness Expo: On January 18 and 19, 2020, NIAAA exhibited at the NBC4/Telemundo Health and Fitness Expo in Washington, D.C. An estimated 70,000 people attend this annual event. NIAAA had an exhibit booth offering general information about alcohol and health, as well as the NIAAA Treatment Navigator. As part of this year’s booth package, NIAAA was able to benefit from a partnership NBC4 had formed with Quest Diagnostics to show video public service announcements (PSAs) on television screens inside public waiting rooms at Quest facilities. During the two weeks leading up to the Expo, a 30-second PSA promoting the NIAAA Alcohol Treatment Navigator was shown on TV monitors in 47 waiting rooms located in the local D.C. area, with an estimated 150,000 impressions. Watch the PSA.
Partnerships, Outreach, and Public Liaison Activities
Alcohol Awareness Month
NIAAA outreach activities as part of Alcohol Awareness Month during April 2020 included:
- Social media posts on Twitter (@NIAAAnews) and Instagram (@NIAAAnews)
- Alcohol Awareness Month Digital Marketing Campaign: NIAAA Communications developed focused posts for Google, Twitter, Facebook, and Instagram informing audiences about fact sheets on alcohol overdose (in Spanish and English) and hangovers, in addition to developing two Twitter polls on these topics
- Twitter chats:
- American Psychiatric Association (APA) on April 29: Mental health and addiction amid the COVID-19 crisis. Dr. Aaron White served as our scientific expert for the chat. Retweets and other social media engagement included liaison groups such as Recover Alaska, Alaska Children’s Trust, CADCA, and SADD.
- American Society of Addiction Medicine (ASAM) on April 30: COVID-19 and social distancing effects on people with alcohol use disorder. Dr. Aaron White served as our scientific expert. Participating through retweets and other social media engagement were various professional groups such as the American College of Obstetricians and Gynecologists (ACOG), the ATTC Network, and Alcoholism & Drug Abuse Weekly News (ADAW); as well as government Twitter feeds belonging to HHS, FDA, NIH, and SAMHSA.
NIAAA Seasonal Outreach
Super Bowl Safety Awareness: NIAAA tweeted several Super Bowl safety messages on January 31 and February 1, 2020, resulting in 79 engagements (likes, retweets, replies, and URL clicks). Other stakeholder groups were also encouraged to disseminate the message on their social media channels.
Additional media coverage:
• The New York Times ran a first-person story, “What Does It Mean to Have a Serious Drinking Problem?” on Super Bowl weekend.
• Maine Public Radio, Yahoo News, and Healthline.com and posted articles that included the NIAAA Alcohol Treatment Navigator and the NIAAA website as resources.
• Valentine’s Day Health Message: NIAAA tweeted a Valentine’s Day health message to “Love Your Liver” on February 14, 2020. St. Patrick’s Day Safety Awareness: NIAAA tweeted a St. Patrick’s Day safety message on March 17, 2020.
Select Partnership Updates
- ACHA Publications: NIAAA collaborated with the American College Health Association (ACHA) to promote the NIAAA fact sheet on alcohol overdose.
Web Updates
-
COVID-19 Related Information: In April, NIAAA rolled out a new a web resource: Fact Sheet – Alcohol Treatment and Physical Distancing. The material spotlights telehealth options and virtual resources to find professionally led treatment and mutual-support groups online. In addition, NIAAA inserted new Frequently Asked Questions for visitors to the Alcohol Treatment Navigator website.
-
Home Page Refresh—Initial Phase: As part of the first phase of an ongoing effort to update and improve the NIAAA Main website, the web team recently launched new design elements on the home page. The refresh features improved graphics and navigation with quick links to popular web resources. The web team continues to work on a refresh for the rest of the website, due to launch within this calendar year.

