NIAAA Director's Report on Institute Activities to the 148th Meeting of the National Advisory Council on Alcohol Abuse and Alcoholism
Table of Contents
- NIAAA BUDGET
- COLLABORATIVE RESEARCH ON ADDICTION AT NIH UPDATE
- DIRECTOR'S ACTIVITIES
- STAFF TRANSITIONS
- HONORS & AWARDS
- NEW REQUESTS FOR APPLICATIONS AND PROGRAM ANNOUNCEMENTS
- NOTABLE NIAAA STAFF ACTIVITIES
- NIAAA RESEARCH HIGHLIGHTS
- NIAAA COMMUNICATIONS ACTIVITIES
NIAAA BUDGET
Fiscal Year (FY) 2017
NIAAA closed out FY 2017 on September 30, 2017; the final appropriation for NIAAA was $483.4 million.
FY 2018
On March 23, 2018, the President signed H.R.1625 – Consolidated Appropriations Act, 2018. NIH received a total of $37 billion, $3 billion above the FY 2017 enacted level. This funding includes specific increases for research on opioids, Alzheimer’s disease, antimicrobial resistance, and influenza. The bill provides a general increase to all NIH Institutes and Centers (ICs) and continues support for the Gabriella Miller Kids First Act pediatric research initiative.
The FY 2018 appropriation for NIAAA provides $509.6 million. This represents an increase of $27.2 million or a 5.6 percent increase over the FY 2017 budget level. NIAAA estimates it will support a total of 752 research project grants (RPGs) in FY 2018 compared to 682 for FY 2017.
FY 2019
The President released his FY 2019 budget, including an addendum, in February, requesting $33 billion for NIH, a $1 billion decrease from FY 2017, and $469.1 million for NIAAA, a $13.4 million decrease from FY 2017.
COLLABORATIVE RESEARCH ON ADDICTION AT NIH (CRAN) UPDATE
Adolescent Brain and Cognitive Development (ABCD) Study
The ABCD Study (https://abcdstudy.org/) continues to meet enrollment targets and is now focusing on including subjects from population subgroups in sufficient numbers to allow the best opportunity for analyses. To date, more than 9,000 youth and their families have been recruited for the study, which is on target to meet the overall recruitment goal.
In late February 2018, in accordance with the commitment of NIAAA and the National Institute on Drug Abuse to make the ABCD Study data available to the widest possible number of researchers as soon as possible, the first curated data release was announced. Approximately 30 terabytes of data obtained from the first 4,500 participants were made available to scientists worldwide to conduct research on the many factors that influence brain, cognitive, social, and emotional development. This interim release provides high-quality baseline data on the sample of 9- to 10-year-old children, including basic participant demographics, assessments of physical and mental health, substance use, culture and environment, neurocognition, tabulated structural and functional neuroimaging data, and minimally processed brain images, as well as biological data such as pubertal hormone analyses. The data are made available through the National Institute of Mental Health (NIMH) Data Archive (https://data-archive.nimh.nih.gov/abcd) and can be accessed by researchers who obtain a free NIMH Data Archive account (https://data-archive.nimh.nih.gov/abcd/request-access). All personally identifiable information is removed from the data to ensure participant confidentiality and anonymity.
DIRECTOR’S ACTIVITIES
NIAAA’s Director, George F. Koob, Ph.D., made several important presentations presentations between January 1, 2018, and March 31, 2018.
- He gave an NIAAA intramural research seminar in Rockville, Maryland, on January 18, 2018, titled “Compulsive Drug Seeking: A Negative Reinforcement View.”
- He presented to the Brain Science Research Consortium Unit Special Grand Rounds at the University of Maryland School of Medicine, Baltimore, Maryland, on February 1, 2018. His talk was titled “Neurobiology of Addiction: The Gain in the Brain is in the Pain.”
- He presented at the Community Anti-Drug Coalitions of America National Leadership Forum at National Harbor, Maryland, on February 6, 2018. His talk was titled “NIAAA Update: Progress and Priorities.”
- He presented at Yale University, New Haven, Connecticut, on February 21, 2018. His talk was titled “What Science Can Tell You About the Diagnosis, Prevention and Treatment of Alcoholism.”
- He spoke at Brown University in Providence, Rhode Island, on February 22, 2018. His talk was titled “Neurobiology of Addiction: The Gain in the Brain is in the Pain.”
- He presented a seminar at Catholic University of America in Washington, D.C., on February 26, 2018. His talk was titled “The Neurobiology of Addiction: A Negative Reinforcement View.”
- He spoke at the annual meeting of the American Psychopathological Association, in New York, New York, on March 1, 2018. His talk was titled “How the Neurobiology of the Dark Side of Addiction Interfaces with Psychiatric Comorbidities.”
- He spoke at the Gordon Research Conference on Alcohol and the Nervous System in Galveston, Texas, on March 4, 2018. His talk was titled “Neurobiological Contributions to the Diagnosis, Prevention and Treatment of Alcohol Use Disorders: NIAAA Update.”
- He addressed the Friends of NIAAA in Washington, D.C., on March 13, 2018. His talk was titled “Role of Alcohol in the Opioid Epidemic".
STAFF TRANSITIONS
New Staff
 Hemin Chin, Ph.D., joined the NIAAA Division of Neuroscience and Behavior as a Senior Program Director in April 2018. After receiving a Ph.D. in Neurobiology and Physiology from Northwestern University, Dr. Chin came to NIH in 1983 for postdoctoral training in molecular neurobiology in the Laboratory of Biochemical Genetics at the National Heart, Lung, and Blood Institute. He continued independent research in the structure and function of voltage-sensitive calcium channel genes in the Laboratory of Molecular Biology and the Laboratory of Neurochemistry, National Institute of Neurological Disorders and Stroke. He then joined the Division of Neuroscience and Basic Behavioral Science, National Institute of Mental Health, as Chief of the Genetics Basis of Neural Function Program in 1998 and then in 2003 joined the National Eye Institute (NEI), where he served in various positions. Dr. Chin has received several NIH Director’s Awards for his contributions to the NEI mission and to trans-NIH genetics and genomics initiatives, including the NEI Age-Related Eye Disease Study, NEI Database of Genotypes and Phenotypes Group, NEI Age-Related Macular Degeneration Gene Consortium, Trans-NIH Zebrafish Genomic Initiative, Genome-Wide Association Study Data Sharing Policy development, and Genomic Data Sharing Policy oversight and governance. He has organized numerous national and international symposia and meetings, has published more than 50 papers and book chapters, and recently co-edited a book, Methods in Genomic Neuroscience..
Hemin Chin, Ph.D., joined the NIAAA Division of Neuroscience and Behavior as a Senior Program Director in April 2018. After receiving a Ph.D. in Neurobiology and Physiology from Northwestern University, Dr. Chin came to NIH in 1983 for postdoctoral training in molecular neurobiology in the Laboratory of Biochemical Genetics at the National Heart, Lung, and Blood Institute. He continued independent research in the structure and function of voltage-sensitive calcium channel genes in the Laboratory of Molecular Biology and the Laboratory of Neurochemistry, National Institute of Neurological Disorders and Stroke. He then joined the Division of Neuroscience and Basic Behavioral Science, National Institute of Mental Health, as Chief of the Genetics Basis of Neural Function Program in 1998 and then in 2003 joined the National Eye Institute (NEI), where he served in various positions. Dr. Chin has received several NIH Director’s Awards for his contributions to the NEI mission and to trans-NIH genetics and genomics initiatives, including the NEI Age-Related Eye Disease Study, NEI Database of Genotypes and Phenotypes Group, NEI Age-Related Macular Degeneration Gene Consortium, Trans-NIH Zebrafish Genomic Initiative, Genome-Wide Association Study Data Sharing Policy development, and Genomic Data Sharing Policy oversight and governance. He has organized numerous national and international symposia and meetings, has published more than 50 papers and book chapters, and recently co-edited a book, Methods in Genomic Neuroscience..

Studly Auguste joined the Office of the Clinical Director as Clinical Operations Manager on April 2, 2018. Previously, she worked in the Administrative Management Branch of the National Eye Institute and the Cancer Prevention Fellowship Program at the National Cancer Institute.
 Csaba Matyas, Ph.D., joined the NIAAA Laboratory of Cardiovascular Physiology and Tissue Injury in May 2018 as a Postdoctoral Visiting Fellow. He received his Ph.D. in Theoretical Medicine from Semmelweis University, Hungary, in October 2017. His doctoral training focused on the topic of the “Role of Nitrooxidative Stress and No-cGMP Signaling in the Development of Cardiovascular Disorders and Therapy.” At NIAAA, Dr. Matyas will perform hemodynamic measurements in mice and rats to study the effects of alcohol and other substances of abuse. He will also be working on the development of new pressure-volume catheters for hemodynamic measurements and models of tissue injury in mice and rats, learning various molecular biology and biochemistry methods to evaluate consequences of tissue injury, and testing new drug targets against inflammation and tissue injury.
Csaba Matyas, Ph.D., joined the NIAAA Laboratory of Cardiovascular Physiology and Tissue Injury in May 2018 as a Postdoctoral Visiting Fellow. He received his Ph.D. in Theoretical Medicine from Semmelweis University, Hungary, in October 2017. His doctoral training focused on the topic of the “Role of Nitrooxidative Stress and No-cGMP Signaling in the Development of Cardiovascular Disorders and Therapy.” At NIAAA, Dr. Matyas will perform hemodynamic measurements in mice and rats to study the effects of alcohol and other substances of abuse. He will also be working on the development of new pressure-volume catheters for hemodynamic measurements and models of tissue injury in mice and rats, learning various molecular biology and biochemistry methods to evaluate consequences of tissue injury, and testing new drug targets against inflammation and tissue injury.
Internal NIAAA Transitions
 Bridget Williams-Simmons, Ph.D., has been appointed as the Director of the NIAAA Office of Science Policy and Communications. Dr. Williams-Simmons joined NIAAA in 2007 as a Health Scientist Administrator in the Science Policy Branch (SPB), where she worked on a wide range of science policy topics. During her tenure in SPB, she served as NIAAA’s American Recovery and Reinvestment Act coordinator as well as NIAAA lead on a number of other significant trans-NIH projects. Prior to her recent appointment, Dr. Williams-Simmons served as Chief of SPB. She received her Ph.D. in Biochemistry from Tulane University.
Bridget Williams-Simmons, Ph.D., has been appointed as the Director of the NIAAA Office of Science Policy and Communications. Dr. Williams-Simmons joined NIAAA in 2007 as a Health Scientist Administrator in the Science Policy Branch (SPB), where she worked on a wide range of science policy topics. During her tenure in SPB, she served as NIAAA’s American Recovery and Reinvestment Act coordinator as well as NIAAA lead on a number of other significant trans-NIH projects. Prior to her recent appointment, Dr. Williams-Simmons served as Chief of SPB. She received her Ph.D. in Biochemistry from Tulane University.

Jennifer A. Hobin, Ph.D., has been appointed as the Chief of the NIAAA Science Policy Branch. Dr. Hobin joined NIAAA as a Senior Health Science Policy Analyst in 2014. She came to NIAAA from the American Association for Cancer Research (AACR), where she served as the Director of Science Policy. Prior to AACR, she served as the Director of Science Policy at the Federation of American Societies for Experimental Biology, where she worked on a broad range of policy issues in support of the nation’s biomedical research enterprise. She received her Ph.D. in Biopsychology from the University of Michigan.
Departing Staff

Sofia Bouhlal, Ph.D., who was a Visiting Fellow in the Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology (CPN), has returned to France to be with her family. She remains an active collaborator with the CPN team.

Gayle McCrossin, who served as an inpatient Nurse Practitioner with the NIAAA Office of the Clinical Director, has taken an equivalent position with the Rehabilitation Medicine Department of the NIH Clinical Center.
HONORS & AWARDS
Dr. George F. Koob was elected to the National Academy of Medicine. See news release at: https://www.niaaa.nih.gov/news-events/news-releases/george-f-koob-phd-elected-national-academy-medicine.
Dr. Mehdi Farokhnia, Postdoctoral Fellow in the Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology, was selected as a speaker (poster selected for oral communication) at the Alcohol & Nervous System Gordon Research Seminar in Galveston, Texas, in March 2018.
NEW REQUESTS FOR APPLICATIONS (RFAs) AND PROGRAM ANNOUNCEMENTS (PAs)
Notice of Funding Opportunities (NOFO) Issued by NIAAA
Genetics of Alcohol Sensitivity and Tolerance (R01 – Clinical Trial Not Allowed) PA-18-660; (R21/R33 – Clinical Trial Not Allowed) PAR-18-659. This PA solicits applications on novel genetic mechanisms underlying the development of tolerance and the progression to alcohol use disorder (AUD).
Supplements to Advance Research (STAR) from Projects to Programs (Admin Supp – Clinical Trial Optional) PA-18-647. This PA solicits administrative supplement applications to promote innovation and novel high-risk projects by early established investigators (EEIs). The supplement will allow EEIs to expand and explore new opportunities within the scope of the currently funded R01 grant, and to facilitate the transition from a single, structured research project to a research program.
Understanding Processes of Recovery in the Treatment of Alcohol Use Disorder (R01 – Clinical Trial Optional) PA-18-619; (R21 – Clinical Trial Optional) PA-18-620. This PA solicits applications that examine processes of recovery and relapse in the treatment of AUD, focusing on 1) defining recovery; 2) Examining new and innovative methods to examine precipitants of relapse; 3) Understanding mechanisms of mutual help and recovery; 4) Evaluating recovery systems of care; and 5) Examining processes of extended treatment for AUD.
NIH-Wide NOFOs with NIAAA’s Participation
Addressing the Challenges of the Opioid Epidemic in Minority Health and Health Disparities Research in the U.S. (R21 – Clinical Trial Optional) PAR-18-745; (R01 – Clinical Trial Optional) PAR-18-747.
Alzheimer’s Disease and its related Dementias (AD/ADRD)-Focused Administrative Supplements for NIH Grants that Are Not Focused on Alzheimer’s Disease (All Mechanisms) NOT-AG-18-008.
BRAIN Initiative: Targeted BRAIN Circuits Projects (R01 – Clinical Trial Not Allowed) RFA-NS-18-030.
BRAIN Initiative: New Technologies and Novel Approaches for Large-Scale Recording and Modulation in the Nervous System (R01 – Clinical Trial Not Allowed) RFA-NS-18-020.
BRAIN Initiative: Optimization of Transformative Technologies for Large-Scale Recording and Modulation in the Nervous System (U01 – Clinical Trial Not Allowed) RFA-NS-18-019.
BRAIN Initiative: New Concepts and Early-Stage Research for Large-Scale Recording and Modulation in the Nervous System (R21 – Clinical Trial Not Allowed) RFA-EY-18-001.
Fogarty HIV Research Training Program for Low- and Middle-Income Country Institutions (D43 – Clinical Trial Optional) PAR-18-717.
Improving Patient Adherence to Treatment and Prevention Regimens to Promote Health (R01 – Clinical Trial Optional) PA-18-722; (R21 – Clinical Trial Optional) PA-18-723.
Improving Patient Adherence to Treatment and Prevention Regimens to Promote Health Interdisciplinary Research Teams to Investigate Reciprocal Basic Behavioral and Social Linkages Between Sleep and Stress (R24 – Clinical Trial Optional) PAR-18-694.
Small Research Grants for Establishing Basic Science-Clinical Collaborations to Understand Structural Birth Defects (R03 – Clinical Trial Not Allowed) PAR-18-734.
Small Research Grants for Analyses of Data for the Gabriella Miller Kids First Data Resource (R03 – Clinical Trial Not Allowed) PAR-18-733.
Women and Sex/Gender Differences in Drug and Alcohol Abuse/Dependence (R03 – Clinical Trial Optional) PA-18-601; (R21 – Clinical Trial Optional) PA-18-602; (R01 – Clinical Trial Optional) PA-18-603.
NOTABLE NIAAA STAFF ACTIVITIES -- JANUARY 2018 - MARCH 2018
NIAAA held a workshop titled “Clinical Trial Design and Endpoints for Alcoholic Hepatitis (AH) and other Alcohol-Associated Liver Diseases (AALD),” on March 26 and 27, 2018, in Silver Spring, Maryland. This two-day workshop was a trans-NIH effort in collaboration with the U.S. Food and Drug Administration and the American Association for the Study of Liver Diseases. The purpose of the workshop was to develop recommendations for standardized definitions, variable sets, screening and assessment tools, and procedures to advance clinical research and drug development in AH and AALD. In addition, the workshop included presentations on alcohol addiction and treatment, which is a new and emerging topic for the hepatology field.
- Dr. Svetlana Radaeva co-organized the workshop;
- Dr. Bridget Williams-Simmons served on the Steering Committee;
- Dr. M. Katherine Jung made introductory remarks;
- Dr. George F. Koob co-chaired the program’s first session, titled “Overview of AH and AALD: What Do We Know? Where Are the Gaps?”;
- Dr. Raye Litten presented “Treatment of Alcohol Use Disorder”;
- Dr. Daniel Falk presented “Alcohol-Related Instruments and Endpoints Used in Alcohol Clinical Trials”;
- Dr. Lori Ducharme presented “Linking Patients with Community-Based Treatment for Alcohol Use Disorder”; and
- Drs. Lorenzo Leggio and Mary Lee served as discussants for the session titled “Approach to Safety and Supportive Care.”
Dr. Margaret (Peggy) Murray and Joan Romaine organized a Foundation for Advanced Education in Sciences at the NIH graduate-level course, “Alcohol Across the Lifespan,” a 15-week course for spring 2018. The course comprised the following lectures:
- Dr. Peggy Murray provided a lecture on an “Overview of the Course” that included “Epidemiology and Definitions of Terms” and showing the film Risky Drinking.
- Dr. George F. Koob provided a lecture on “Neurobiology of Addiction.”
- Dr. David Goldman provided a lecture on “Genes of Addiction: How Many, and How They Work.”
- Dr. Kenneth Warren provided a lecture on “Embryo/Fetus and Infancy.”
- Drs. Joshua Gowin and Aaron White provided lectures on “Children, Young Adolescents, and Youth.”
- Dr. Ralph Hingson provided a lecture on “Young Adults/College Students/Military.”
- Dr. M. Katherine Jung provided a lecture on “Health Effects of Alcohol.”
- Drs. Bin Gao and Lorenzo Leggio provided a lecture on “Liver Disease.”
- Drs. Brett Hagman and Lori Ducharme provided a lecture on “Diagnosis and Treatment of Alcohol Use Disorder,” including a presentation of the NIAAA Treatment Navigator.
- Dr. Lorenzo Leggio provided a lecture on “Medication-Assisted Treatment of Alcohol Use Disorder.”
- Dr. Judith Arroyo provided a lecture on “Alcohol Problems in Diverse Populations.”
- Dr. Deidra Roach provided a lecture on “Alcohol and Women.”
- Dr. Falk Lohoff provided a lecture on “Co-Morbidities, PTSD.”
Dr. Judith Arroyo demonstrated the Native Communities Alcohol Intervention Review (NativeAIR) to the NIH Tribal Advisory Committee (TAC), as part of the semi-annual government-to-government consultation meetings between NIH and Tribal leaders on March 13, 2018. NativeAIR is a process for reviewing the peer-reviewed literature on alcohol interventions with Native populations and a website (a draft of which was demonstrated) for sharing research information with general audiences and the research community. The demonstration focused on a pilot of the review process conducted on the prevention of fetal alcohol spectrum disorder (FASD) literature in Native American communities. Feedback from the TAC was overwhelmingly positive, and Tribal leaders recommended to Drs. Collins and Tabak that this NIAAA-developed method to abstract and disseminate research information serve as the prototype for other NIH ICs to communicate research-based evidence to Native American communities. NativeAIR will continue to review and disseminate information on the remaining 75–100 interventions and will work toward establishing a webpage and other forms of dissemination.
Dr. Brett Hagman and the Mechanisms of Behavior Change (MOBC) Committee published a special issue in the Journal of Studies on Alcohol and Drugs on innovative topics for understanding MOBC in the treatment of alcohol use disorder (AUD).
Dr. Lori Ducharme presented a session on “Strategies for Finding Evidence-Based Alcohol Treatment” at the 2018 Community Anti-Drug Coalitions of America (CADCA) National Leadership Forum in Washington, D.C., on February 6, 2018.
Dr. Changhai Cui served as discussion leader on “The Role of the Cerebellum in Alcohol Use Disorder” at 2018 Gordon Research Conference (GRC) for Alcohol & the Nervous System, March 4–9, 2018, in Galveston, Texas. She introduced the session and highlighted recent conceptual advances on the role of the cerebellum in cognition, emotion, and addiction. In addition, Drs. Changhai Cui and Antonio Noronha presented NIH Funding Mechanisms and Process focusing on Fellowships and Early Career Development Awards at Alcohol and the Nervous System Gordon Research Seminar, which was held in conjunction with the GRC.
Dr. Mariela Shirley was a speaker at the annual Collaborative Perspectives on Addictions Meeting on March 15, 2018, in Tampa, Florida. She presented a workshop titled “Building a Successful NIH Grant Application: Connecting Scientific Priorities and Policies.” The meeting was organized by the Society of Addiction Psychology (Division 50) of the American Psychological Association.
Dr. Lorenzo Leggio gave a seminar at Columbia University/New York State Psychiatric Institute in New York, New York, in February 2018, titled “More than a Gut Feeling: The Role of Gut-Brain Neuroendocrine Pathways in Alcohol Use Disorder.”
NIAAA RESEARCH HIGHLIGHTS
Digoxin Suppresses Pyruvate Kinase M2-Promoted HIF-1A Transactivation in Steatohepatitis
Significance: Hypoxia is a common feature observed in animal models of alcohol-induced liver disease and, more recently, in the liver of human binge drinkers. A highly conserved host-protective response to hypoxia is orchestrated by the transcription factor HIF-1 alpha. A persistent HIF-1 alpha response to hypoxia in a host, similar to chronic inflammation, is thought to contribute to the development of many medical conditions, including alcohol-associated liver diseases (AALD). The current study provides further evidence supporting this concept and also demonstrates the potential of digoxin, a widely used medication for heart disease, in treatment of AALD via the HIF-1 alpha pathway.
Sterile inflammation after tissue damage is a ubiquitous response, yet it has the highest amplitude in the liver. This has major clinical consequences, for alcoholic and non-alcoholic steatohepatitis (ASH and NASH) account for the majority of liver disease in industrialized countries and both lack therapy. Requirements for sustained sterile inflammation include increased oxidative stress and activation of the HIF-1α signaling pathway. We demonstrate the ability of digoxin, a cardiac glycoside, to protect from liver inflammation and damage in ASH and NASH. Digoxin was effective in maintaining cellular redox homeostasis and suppressing HIF-1α pathway activation. A proteomic screen revealed that digoxin binds pyruvate kinase M2 (PKM2), and independently of PKM2 kinase activity results in chromatin remodeling and downregulation of HIF-1α transactivation. These data identify PKM2 as a mediator and therapeutic target for regulating liver sterile inflammation, and demonstrate a novel role for digoxin that can effectively protect the liver from ASH and NASH. (Ouyang X, et al., Mehal WZ. Cell Metab. 2018 Feb 6;27(2):339-350.e3)
Significance: The molecular mechanisms through which binge alcohol-induced gut leakiness contributes to endotoxemia and inflammatory liver disease are poorly understood. This study demonstrated the critical roles that apoptosis of enterocytes (cells of the intestinal lining) and nitration of intestinal junctional complex proteins followed by ubiquitin-dependent proteolytic degradation play in promoting binge alcohol-induced gut leakiness.
Binge alcohol causes gut leakiness, contributing to increased endotoxemia and inflammatory liver injury, although the molecular mechanisms are still elusive. This study was aimed at investigating the roles of apoptosis of enterocytes and nitration followed by degradation of intestinal tight junction (TJ) and adherent junction (AJ) proteins in binge alcohol-induced gut leakiness. The levels of intestinal (ileum) junctional complex proteins, oxidative stress markers and apoptosis-related proteins in rodents, T84 colonic cells and autopsied human ileums were determined by immunoblot, immunoprecipitation, immunofluorescence, and mass-spectral analyses. Binge alcohol exposure caused apoptosis of gut enterocytes with elevated serum endotoxin and liver injury. The levels of intestinal CYP2E1, iNOS, nitrated proteins and apoptosis-related marker proteins were significantly elevated in binge alcohol-exposed rodents. Differential, quantitative mass-spectral analyses of the TJ-enriched fractions of intestinal epithelial layers revealed that several TJ, AJ and desmosome proteins were decreased in binge alcohol-exposed rats compared to controls. Consistently, the levels of TJ proteins (claudin-1, claudin-4, occludin and ZO-1), AJ proteins (β-catenin and E-cadherin) and desmosome plakoglobin were very low in binge-alcohol exposed rats, wild-type mice, and autopsied human ileums but not in Cyp2e1-null mice. Additionally, pretreatment with specific inhibitors of CYP2E1 and iNOS prevented disorganization and/or degradation of TJ proteins in alcohol-exposed T84 colonic cells. Furthermore, immunoprecipitation followed by immunoblot confirmed that intestinal TJ and AJ proteins were nitrated and degraded via ubiquitin-dependent proteolysis, resulting in their decreased levels. These results demonstrated for the first time the critical roles of CYP2E1, apoptosis of enterocytes and nitration followed by ubiquitin-dependent proteolytic degradation of the junctional complex proteins in promoting binge alcohol-induced gut leakiness and endotoxemia, contributing to inflammatory liver disease. (Cho YE, Yu L-R, Abdelmegeed MA, Yoo SH, Song BJ, J Hepatol 2018 Feb 16. (18) 30121-1. [Epub ahead of print])
Significance: This study represents the efforts of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), an interdisciplinary consortium of investigators supported by NIAAA. Using a sample of children enrolled in a prospective CIFASD cohort in Ukraine, the researchers examined the relationship between maternal alcohol consumption, maternal immune profile, and risk of FASD in the offspring. They found that late pregnancy increases in the ratios of two pro-inflammatory cytokines (IL-6 and TNF- α) to the anti-inflammatory cytokine IL-10 had a negative impact on mental development at 12 months of age as well as psychomotor development at 6 and 12 months of age. Furthermore, maternal alcohol use was associated with an increase in cytokine levels throughout pregnancy; however, subjects whose anti-inflammatory IL-10 levels in the third trimester did not increase to maintain a proper balance of pro- and anti-inflammatory cytokines had an elevated risk of having a child with FASD. These results extend findings in animal models and support the premise that an imbalance between pro- and anti-inflammatory maternal cytokines may contribute to neurobehavioral impairments of, and alter the risk for, FASD.
Excessive alcohol consumption has been shown to increase serum plasma levels of numerous immune cytokines. Maternal immune activation and elevated cytokines have been implicated in certain neurological disorders (e.g., autism and schizophrenia) in the offspring. We investigated the hypothesis that elevated cytokines during pregnancy are a risk factor in women who gave birth to a child with Fetal Alcohol Spectrum Disorder (FASD) or a child with neurobehavioral impairment, regardless of prenatal alcohol exposure. Moderate to heavy alcohol-exposed (AE) (N = 149) and low or no alcohol-exposed (LNA) (N = 92) women were recruited into the study during mid pregnancy (mean of 19.8 ± 5.8 weeks' gestation) in two regions of Ukraine: Khmelnytsky and Rivne. Maternal blood samples were obtained at enrollment into the study at early to mid-pregnancy and during a third-trimester follow-up visit and analyzed for plasma cytokines. Children were examined at 6 and/or 12 months of age and were classified as having FASD if their mothers reported alcohol use and if they had at least one standardized score (Bayley Scales of Infant Development II Mental Development Index [MDI], or Psychomotor Development Index [PDI]) below 85 with the presence or absence of physical features of FASD. In multivariate analyses of maternal cytokine levels in relation to infant MDI and PDI scores in the entire sample, increases in the ratio of TNF-α/IL-10 and IL-6/IL-10 were negatively associated with PDI scores at 6 months (p = 0.020 and p = 0.036, respectively) and 12 months (p = 0.043 and p = 0.029, respectively), and with MDI scores at 12 months (p = 0.013 and p = 0.050, respectively). A reduction in the odds ratio of having an FASD child was observed with increasing levels of IL-1β, IL-2, IL-4, IL-6, and IL-10 in early to mid-pregnancy and IL-1β and IL-10 during late pregnancy. However, women that failed to increase IL-10 levels in the third trimester in order to maintain the balance of pro- and anti-inflammatory cytokines had an elevated risk of having an FASD child, specifically a significant increase in the odds ratio of FASD with every one-unit log increase in late pregnancy TNF-α/IL-10 levels (aOR: 1.654, CI: 1.096-2.495, p = 0.017). These data support the concept that disruptions in the balance between pro- and anti-inflammatory cytokines may contribute to neurobehavioral impairment and alter the risk of FASD. (Sowell KD, Uriu-Adams JY, Van de Water J, Chambers CD, Coles CD, Kable JA, Yevtushok L, Zymak-Zakutnya N, Wertelecki W, Keen CL; Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). Alcohol. 2018 May;68:49-58)
Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities
Significance: In this study, May and colleagues used active-case ascertainment, the most reliable approach for estimating the prevalence of FASD, to determine new prevalence estimates among four U.S. communities with varied demographics. They observed prevalence estimates between 1 percent to 5 percent across the four regions. These new estimates are consistently higher than a recent meta-analysis of 6 studies from the United States with a pooled prevalence of 2 percent. Although their findings may not be generalizable to all U.S. communities, they are likely more accurate than previously reported estimates for the United States. Given that children with FASD often go undiagnosed or misdiagnosed, it is important for clinicians and researchers to be aware of the prevalence of FASD.
IMPORTANCE: Fetal alcohol spectrum disorders are costly, life-long disabilities. Older data suggested the prevalence of the disorder in the United States was 10 per 1000 children; however, there are few current estimates based on larger, diverse US population samples. OBJECTIVE: To estimate the prevalence of fetal alcohol spectrum disorders, including fetal alcohol syndrome, partial fetal alcohol syndrome, and alcohol-related neurodevelopmental disorder, in 4 regions of the United States. DESIGN, SETTING, AND PARTICIPANTS: Active case ascertainment methods using a cross-sectional design were used to assess children for fetal alcohol spectrum disorders between 2010 and 2016. Children were systematically assessed in the 4 domains that contribute to the fetal alcohol spectrum disorder continuum: dysmorphic features, physical growth, neurobehavioral development, and prenatal alcohol exposure. The settings were 4 communities in the Rocky Mountain, Midwestern, Southeastern, and Pacific Southwestern regions of the United States. First-grade children and their parents or guardians were enrolled. EXPOSURES: Alcohol consumption during pregnancy. MAIN OUTCOMES AND MEASURES: Prevalence of fetal alcohol spectrum disorders in the 4 communities was the main outcome. Conservative estimates for the prevalence of the disorder and 95% CIs were calculated using the eligible first-grade population as the denominator. Weighted prevalences and 95% CIs were also estimated, accounting for the sampling schemes and using data restricted to children who received a full evaluation. RESULTS: A total of 6639 children were selected for participation from a population of 13 146 first-graders (boys, 51.9%; mean age, 6.7 years [SD, 0.41] and white maternal race, 79.3%). A total of 222 cases of fetal alcohol spectrum disorders were identified. The conservative prevalence estimates for fetal alcohol spectrum disorders ranged from 11.3 (95% CI, 7.8-15.8) to 50.0 (95% CI, 39.9-61.7) per 1000 children. The weighted prevalence estimates for fetal alcohol spectrum disorders ranged from 31.1 (95% CI, 16.1-54.0) to 98.5 (95% CI, 57.5-139.5) per 1000 children. CONCLUSIONS AND RELEVANCE: Estimated prevalence of fetal alcohol spectrum disorders among first-graders in 4 US communities ranged from 1.1% to 5.0% using a conservative approach. These findings may represent more accurate US prevalence estimates than previous studies but may not be generalizable to all communities. (May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Falk D, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE. JAMA 2018 Feb 6;319(5):474-482)
Binge Drinking Above and Below Twice the Adolescent Thresholds and Health-Risk Behaviors
Significance: Previous research indicates that binge drinking thresholds for reaching a blood alcohol level (BAL) of .08% in youth are age- and gender-specific, and are lower than the adult binge thresholds. The current study explored whether drinking at levels at or more than twice the age-/gender-specific binge thresholds for adolescents was associated with increased health-risk behaviors among high-school students, compared with non-binge-drinking adolescents. The study found that 7 percent of high schoolers binged at levels greater than twice the age-/gender-specific thresholds, 9 percent binged at levels that were less than twice the thresholds but more than the binge thresholds, and 14 percent drank less than the binge thresholds. Significantly higher percentages of binge drinkers who consumed alcohol at or greater than twice the age-/gender-specific thresholds reported illegal drug and tobacco use, risky sexual and traffic behaviors, physical fights, suicide, less school-night sleep, and poorer school grades, compared with the other categories of drinkers. The findings suggest that adolescent alcohol-misuse screening should ask about the maximum number of drinks consumed per occasion and the frequency of such consumption. Also, because drinking at levels at or greater than twice the thresholds was a strong predictor of numerous health-risk behaviors, surveillance surveys should investigate which interventions can reduce consumption at these levels as well as related risky behaviors.
BACKGROUND: Underage drinking has been associated with health-risk behaviors: unintentional and unprotected sex; physical and sexual assault; suicide; homicide; traffic and other unintentional injuries; and overdoses. Five drinks consumed over 2 hours by adult males and 4 drinks by adult females typically produce blood alcohol levels (BALs) of ≥0.08%, which the National Institute on Alcohol Abuse and Alcoholism considers binge drinking. Being smaller, young adolescents can reach adult binge-drinking BALs of ≥0.08% with fewer drinks. Previous research indicates boys ages 9 to 13 would reach ≥0.08% with 3 drinks, 4 drinks at ages 14 to 15, and 5 drinks at ages ≥16. For girls, ≥0.08% is reached with ≥3 drinks at ages 9 to 17 and ≥4 drinks at ages ≥18. This study explores whether, among a national sample of high school students, adolescent binge drinking at ≥twice versus <twice the age-/gender-specific thresholds versus nonbinge drinking heightens associations of drinking with health-risk behaviors. METHODS: In 2015, the Youth Risk Behavior Survey asked a national probability sample of 15,624 high school students grades 9 to 12 (response rate 60%) about their past-month drinking and past-month or past-year health-risk behaviors. Logistic regressions with pairwise comparisons examined the association between different drinking levels and selected risk behaviors, adjusting for age, sex, race/ethnicity, and drinking frequency. RESULTS: Seven percent binged ≥twice and 9% <twice the age-/gender-specific thresholds, and 14% drank less than the binge thresholds. Significantly higher percentages of binge drinkers at ≥twice versus <twice the thresholds versus other drinkers reported illegal drug and tobacco use, risky sexual and traffic behaviors, physical fights, suicide, less school-night sleep, and poorer school grades. CONCLUSIONS: Adolescent alcohol misuse screening should query the maximum number of drinks consumed per occasion and frequency of such consumption. State and national surveillance surveys should include those questions to investigate which individual, family, school, community, and policy interventions reduce consumption beyond binge thresholds and related health-risk behaviors. (Hingson R, and Zha, W. Alcohol Clin Exp Res. 2018 April 10. doi: 10.1111/acer.13627)
Significance: These findings suggest that underage college students, who lack ample first-hand experience observing alcohol use among close friends, may rely on social network sites, specifically the “Likes” attached to peers' alcohol-related posts, to estimate injunctive drinking norms. For first-year college students who have not yet initiated drinking, observing peers' alcohol-related posts receive abundant "likes" may increase perceptions of peer approval for risky drinking.
OBJECTIVE: Examine 1) whether observed social reinforcements (i.e., "likes") received by peers' alcohol-related social media posts are related to first-year college students' perceptions of peer approval for risky drinking behaviors; and 2) whether associations are moderated by students' alcohol use status. PARTICIPANTS: First-year university students (N = 296) completed an online survey in September 2014. METHOD: Participants reported their own alcohol use, friends' alcohol use, perceptions of the typical student's approval for risky drinking, and ranked 10 types of social media posts in terms of the relative numbers of "likes" received when posted by peers. RESULTS: Observed social reinforcement (i.e., "likes") for peers' alcohol-related posts predicted perceptions of peer approval for risky drinking behaviors among non-drinking students, but not drinking students. CONCLUSIONS: For first-year college students who have not yet initiated drinking, observing peers' alcohol-related posts to receive abundant "likes" may increase perceptions of peer approval for risky drinking. (Boyle SC, Smith DJ, Earle AM, LaBrie JW.; J Am Coll Health 2018 Feb 6:1-7. Doi: 10.1080/07448481.2018.1431895.)
Significance: Numerous studies have associated the A118G functional genetic polymorphism in the mu-opioid receptor gene (OPRM1) with alcohol consumption and sensitivity; however, larger genome-wide association studies have yet to demonstrate an association between A118G and having a diagnosis of AUD. This study examined the effect of the A118G on alcohol self-administration, subjective response, and craving in a sample of social drinkers; the results indicated no significant effects. Also, analysis of alcohol consumption data in a larger sample of 965 participants of European ancestry found no relationship between OPRM1 genotype and alcohol consumption in participants with or without AUD. These findings suggest that there may not be an association between OPRM1 A118G genotype and alcohol consumption or sensitivity in individuals of European ancestry.
The endogenous opioid system may be involved in the development and maintenance of alcohol use disorder (AUD) and is a target for existing AUD pharmacotherapies. A functional polymorphism of the mu-opioid receptor gene (OPRM1 A118G, rs1799971) may alter the risk of developing AUD. Human laboratory studies have demonstrated that minor allele carriers self-administer more alcohol, show greater sensitivity to alcohol's effects, and exhibit increased alcohol-induced dopamine release. On the other hand, large genome-wide association studies and meta-analyses of candidate gene studies have not found an association between this genotype and alcohol dependence diagnosis. Given this discrepancy, the present study sought to verify whether OPRM1 A118G was associated with alcohol self-administration, subjective response to alcohol, and craving in a sample of 106 social drinkers of European ancestry who completed an intravenous alcohol self-administration session. We found no relationship between OPRM1 rs1799971 genotype and subjective response to alcohol or craving. OPRM1 genotype was not associated with total alcohol exposure or likelihood of attaining a binge-level exposure (80 mg%) during the intravenous alcohol self-administration session. Analysis of 90-day Timeline Followback interview data in a larger sample of 965 participants of European ancestry found no relationship between OPRM1 genotype and alcohol consumption in either alcohol dependent or non-dependent participants. These findings suggest that there may not be an association between OPRM1 rs1799971 genotype and alcohol consumption or sensitivity in individuals of European ancestry. (Sloan ME, Klepp TD, Gowin JL, Swan JE, Sun H, Stangl BL, Ramchandani VA. Neuropsychopharmacology. 2018 Feb 2. doi: 10.1038/s41386-017-0002-8. [Epub ahead of print])
Advancing Analytic Approaches to Address Key Questions in Mechanisms of Behavior Change Research
Significance: Over the past two decades, there has been considerable interest in understanding the mechanisms of behavior change (MOBC) that underlie how behavioral treatments for AUD work. Mediation analysis frameworks have been the primary approach used to evaluate MOBC, but a greater understanding of how MOBC operate requires the application of advanced analytic techniques that consider the dynamic and non-linear change processes that take place in AUD treatment. This article highlights and reviews several statistical approaches (i.e., growth curve modeling, time-varying effect modeling, moderated mediation, and dynamic-systems modeling) that may enhance our understanding of MOBC in AUD treatment.
OBJECTIVE: Interest in studying mechanisms of behavior change (MOBCs) in substance use disorder (SUD) treatments has grown considerably in the past two decades. Much of this work has focused on identifying which variables statistically mediate the effect of SUD treatments on clinical outcomes. However, a fuller conceptualization of MOBCs will require greater understanding of questions that extend beyond traditional mediation analysis, including better understanding of when MOBCs change during treatment, when they are most critical to aiding the initiation or maintenance of change, and how MOBCs themselves arise as a function of treatment processes. METHOD: In the present study, we review why these MOBC-related questions are often minimally addressed in empirical research and provide examples of data analytic methods that may address these issues. We highlight several recent studies that have used such methods and discuss how these methods can provide unique theoretical insights and actionable clinical information. Results: Several statistical approaches can enhance the field’s understanding of the timing and development of MOBCs, including growth curve modeling, time-varying effect modeling, moderated mediation analysis, dynamic systems modeling, and simulation methods. CONCLUSIONS: Adopting greater diversity in methods for modeling MOBCs will help researchers better understand the timing and development of key change variables and will expand the theoretical precision and clinical impact of MOBC research. Advances in research design, measurement, and technology are key to supporting these advances. (Hallgren KA, Wilson AD, Witkiewitz K J Stud Alcohol Drugs 2018; 79: 182–189)
Significance: The hormone ghrelin is known for its role in regulating appetite and food intake, and previous studies have shown that ghrelin also involved in the brain's reward and stress pathways. The current study describes the development of a novel transgenic rat model lacking the ghrelin receptor that will enable future research on the role of the ghrelin system in substance use disorders and other behaviors.
BACKGROUND/OBJECTIVES: Ghrelin, a stomach-derived hormone implicated in numerous behaviors including feeding, reward, stress, and addictive behaviors, acts by binding to the growth hormone secretagogue receptor (GHSR). Here, we present the development, verification, and initial characterization of a novel GHSR knockout (KO) Wistar rat model created with CRISPR genome editing. METHODS: Using CRISPR/Cas9, we developed a GHSR KO in a Wistar background. Loss of GHSR mRNA expression was histologically verified using RNAscope in wild-type (WT; n = 2) and KO (n = 2) rats. We tested the effects of intraperitoneal acyl-ghrelin administration on food consumption and plasma growth hormone (GH) concentrations in WT (n = 8) and KO (n = 8) rats. We also analyzed locomotion, food consumption, and body fat composition in these animals. Body weight was monitored from early development to adulthood. RESULTS: The RNAscope analysis revealed an abundance of GHSR mRNA expression in the hypothalamus, midbrain, and hippocampus in WTs, and no observed probe binding in KOs. Ghrelin administration increased plasma GH levels (p = 0.0067) and food consumption (p = 0.0448) in WT rats but not KOs. KO rats consumed less food overall at basal conditions and weighed significantly less compared with WTs throughout development (p = 0.0001). Compared with WTs, KOs presented higher concentrations of brown adipose tissue (BAT; p = 0.0322). CONCLUSIONS: We have verified GHSR deletion in our KO model using histological, physiological, neuroendocrinological, and behavioral measures. Our findings indicate that GHSR deletion in rats is not only associated with a lack of response to ghrelin, but also associated with decreases in daily food consumption and body growth, and increases in BAT. This GHSR KO Wistar rat model provides a novel tool for studying the role of the ghrelin system in obesity and in a wide range of medical and neuropsychiatric disorders. (Zallar LJ, Tunstall BJ, Richie CT, Zhang YJ, You ZB, Gardner EL, Heilig M, Pickel J, Koob GF, Vendruscolo LF, Harvey BK, Leggio L. Int J Obes (Lond). 2018 Jan 30 [Epub ahead of print])
Significance: This study supports the therapeutic potential of cannabidiol (CBD), a non-psychoactive constituent of cannabis, in preventing alcohol relapse. Using animal models, the researchers demonstrated beneficial effects of CBD on drug-seeking behavior, anxiety, and impulsivity, as well as long-lasting effects with only brief treatment. The results suggest that CBD can target concurrent behaviors associated with relapse risk, which may be more effective than targeting a single behavior.
BACKGROUND: Cannabidiol (CBD), the major non-psychoactive constituent of Cannabis sativa, has received attention for therapeutic potential in treating neurologic and psychiatric disorders. Recently, CBD has also been explored for potential in treating drug addiction. Substance use disorders are chronically relapsing conditions and relapse risk persists for multiple reasons including craving induced by drug contexts, susceptibility to stress, elevated anxiety, and impaired impulse control. Here, we evaluated the “anti-relapse” potential of a transdermal CBD preparation in animal models of drug seeking, anxiety and impulsivity. Rats with alcohol or cocaine self-administration histories received transdermal CBD at 24 h intervals for 7 days and were tested for context and stress-induced reinstatement, as well as experimental anxiety on the elevated plus maze. Effects on impulsive behavior were established using a delay-discounting task following recovery from a 7-day dependence-inducing alcohol intoxication regimen. CBD attenuated context-induced and stress-induced drug seeking without tolerance, sedative effects, or interference with normal motivated behavior. Following treatment termination, reinstatement remained attenuated up to ≈5 months although plasma and brain CBD levels remained detectable only for 3 days. CBD also reduced experimental anxiety and prevented the development of high impulsivity in rats with an alcohol dependence history. The results provide proof of principle supporting potential of CBD in relapse prevention along two dimensions CBD: beneficial actions across several vulnerability states, and long-lasting effects with only brief treatment. The findings also inform the ongoing medical marijuana debate concerning medical benefits of non-psychoactive cannabinoids and their promise for development and use as therapeutics. (Gonzalez-Cuevas G, Martin-Fardon R, Kerr TM, Stouffer DG, Parsons LH, Hammell DC, Banks SL, Stinchcomb AL, Weiss F Neuropsychopharmacology 2018. doi:10.1038/s41386-018-0050-8).
The Role of Aging, Drug Dependence, and Hepatitis C Comorbidity in Alcoholism Cortical Compromise
Significance: Alcohol misuse over time can have deleterious effects on brain structure and function. Comorbid factors such as drug dependence or hepatitis C infection may also play a role. An often-overlooked comorbid factor is the aging process. The current study examined changes in regional brain volumes in alcohol-dependent individuals and age-matched controls ages 25 to 75 who received one or more magnetic resonance imaging (MRI) scans over a 14-year period. Individuals in the alcohol-dependent group had significant age-related changes, most prominently in the frontal cortex, beyond the normal aging effects seen in matched controls. Drug dependence or hepatitis C infection compounded the alcohol-related effects. These findings suggest that alcohol misuse contributes to accelerated age-related impairments in brain function.
IMPORTANCE: The prevalence of alcohol misuse increased substantially over a decade in adults, particularly in those aged 65 years or older. Ramifications for brain structural integrity are significant, especially in older adults. OBJECTIVES: To combine cross-sectional, longitudinal data to test age-alcoholism interactions and examine the association between prevalent comorbidities (drug dependence and hepatitis C virus [HCV] infection) and cortical volume deficits in alcohol dependence. DESIGN, SETTING, AND PARTICIPANTS: During 14 years, 826 structural magnetic resonance images were acquired in 222 individuals with alcohol dependence and 199 age-matched control participants (aged 25-75 years at initial study), parcellated with a common atlas, and adjusted for brain volume. Longitudinal data were available on 116 participants with alcoholism and 96 control participants. DSM-IV criteria determined alcohol and drug diagnoses; serology testing determined HCV status. The study was conducted at SRI International and Stanford University School of Medicine from April 11, 2003, to March 3, 2017. MAIN OUTCOMES AND MEASURES: Magnetic resonance imaging-derived regional cortical volumes corrected for supratentorial volume and sex. RESULTS: Of the 222 participants with alcoholism, 156 (70.3%) were men; mean (SD) age was 48.0 (10.0) years; the mean age for the 199 control participants was 47.6 (14.0) years. Participants with alcohol dependence had volume deficits in frontal (t = -5.732, P < .001), temporal (t = -3.151, P = .002), parietal (t = -5.063, P < .001), cingulate (t = -3.170, P = .002), and insular (t = -4.920, P < .001) cortices; deficits were prominent in frontal subregions and were not sex dependent. Accelerated aging occurred in frontal cortex (t = -3.019, P < .02) and precentral (t = -2.691, P < .05) and superior gyri (t = -2.763, P < .05) and could not be attributed to the amount of alcohol consumed, which was greater in younger-onset than older-onset participants with alcoholism (t = 6.1191, P < .001). Given the high drug-dependence incidence (54.5%) in the alcoholism group, analysis examined drug subgroups (cocaine, cannabis, amphetamines, opiates) compared with drug-dependence-free alcoholism and control groups. Although the alcohol plus cocaine (t = -2.310, P = .04) and alcohol plus opiate (t = -2.424, P = .04) groups had smaller frontal volumes than the drug-dependence-free alcoholism group, deficits in precentral (t = -2.575, P = .01), supplementary motor (t = -2.532, P = .01), and medial (t = -2.800, P = .01) volumes endured in drug-dependence-free participants with alcoholism compared with control participants. Those with HCV infection had greater deficits than those without HCV infection in frontal (t = 3.468, P = .01), precentral (t = 2.513, P = .03), superior (t = 2.533, P = .03), and orbital (t = 2.506, P = .03) volumes, yet total frontal (t = 2.660, P = .02), insular (t = 3.526, P = .003), parietal (t = 2.414, P = .03), temporal (t = 3.221, P = .005), and precentral (t = 3.180, P = .01) volume deficits persisted in the uninfected participants with alcoholism compared with control participants with known HCV status. CONCLUSIONS AND RELEVANCE: Drug dependence and HCV infection compounded deleterious effects of alcohol dependence on frontal cortical volumes but could not account for the frontally distributed volume deficits in the drug-free participants with alcoholism. We speculate that age-alcohol interactions notable in frontal cortex put older adults at heightened risk for age-associated neurocompromise even if alcohol misuse is initiated later in life. (Sullivan EV, Zahr NM, Sassoon SA, Thompson WK, Kwon D, Pohl KM, Pfefferbaum A. JAMA Psychiatry. [Epub ahead of print])
Working Memory Training Improves Alcohol User’s Episodic Future Thinking: A Rate-Dependent Analysis
Significance: One of the hallmarks of addictive behavior is the inability to postpone immediate rewards (e.g., consuming the drink in front of you) and to discount or not select potentially larger rewards that occur later in time, also known as delay discounting. The current study used working memory training in alcohol-dependent individuals to reduce delay discounting and expand their temporal window for future rewards. Working memory training significantly improved episodic future thinking in alcohol-dependent individuals who had the highest discounting rates at baseline. This study suggests that working memory training can have the beneficial effect of improving the ability to postpone immediate awards in individuals with alcohol dependence.
BACKGROUND: Episodic thinking, whether past or future, uses similar neural machinery, and individuals with alcohol dependence have clear challenges with both. Moreover, alcohol-dependent individuals' narrowed temporal window likely gives rise to greater valuation of immediate rewards. We aimed to strengthen working memory (WM) in alcohol-dependent individuals and measure performance on near-transfer (novel WM) and far-transfer delay discounting (DD) tasks, including episodic future thinking (EFT) performance. Importantly, heterogeneous intervention responses could obscure a treatment effect due to individuals' baseline differences. Therefore, we considered WM, DD, and EFT DD scores using rate-dependent analyses. METHODS: A total of 50 alcohol-dependent individuals received either 20 active (Trained) or sham (Control) WM training sessions using the Cogmed adaptive WM training program. Participants completed a near-transfer novel WM task and far-transfer DD and EFT DD tasks before and after training. RESULTS: Active WM training improved performance on the near-transfer task. As determined by Oldham's correlation [rmean(x,y), y - x], initially low near-transfer task scores improved more than initially high scores (i.e., rate dependence) in the Trained group only. Moreover, Trained group individuals with the highest rates of EFT DD at baseline rate-dependently decreased following training, whereas WM training had no effect on DD alone. CONCLUSIONS: These data support the notion that WM training improves near-transfer task performance and may enhance the effects of EFT DD in a subset of alcohol-dependent individuals trapped within the narrowest temporal window. Rate-dependent changes highlight that we should attend to baseline performance to better identify individuals who would most benefit from an intervention. (Snider SE, Deshpande HU, Lisinski JM, Koffamus MN, LaConte SM, Bickel WK. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2018; 3:160-167)
Press and Publications Activities:
Recent News Media Interviews: Dr. George F. Koob continues to speak with a variety of national and international news outlets on timely topics related to NIAAA’s research and its impact on treatment and prevention of alcohol misuse and AUD. Notable interviews since February 2018 include those with The New York Times, Medscape, The Washington Times, MensHealth.com, Scientific American, and PBS NewsHour.
Drs. Lori Ducharme and Aaron White appeared on National Public Radio’s 1A program in an hour-long discussion titled “The United States of Alcohol Abuse” on January 9, 2018.
Press Release: Study of first-graders shows fetal alcohol spectrum disorders prevalent in US communities (February 6, 2018). Press release available here: https://www.nih.gov/news-events/news-releases/study-first-graders-shows-fetal-alcohol-spectrum-disorders-prevalent-us-communities. 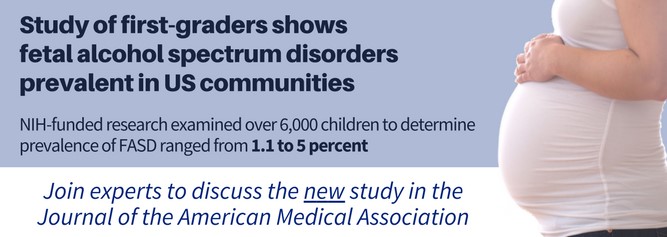 The NIAAA-supported study “Prevalence of Fetal Alcohol Spectrum Disorders in Four Communities of the United States: Results from the Collaboration on Fetal Alcohol Spectrum Disorders Prevalence (CoFASP)” was published in JAMA in early February. The NIAAA press release was distributed pre-embargo via EurekAlert. The study received wide press coverage, with articles in The New York Times, Medscape, and The Washington Times, as well as on WBUR and other outlets. The finding was also featured in the February 13 issue of NIH Research Matters. NIAAA communications staff arranged a post-embargo telebriefing for stakeholders and federal partners, which attracted more than 130 callers. To hear the complete teleconference, visit https://www.youtube.com/watch?v=n1NiekmT828.
The NIAAA-supported study “Prevalence of Fetal Alcohol Spectrum Disorders in Four Communities of the United States: Results from the Collaboration on Fetal Alcohol Spectrum Disorders Prevalence (CoFASP)” was published in JAMA in early February. The NIAAA press release was distributed pre-embargo via EurekAlert. The study received wide press coverage, with articles in The New York Times, Medscape, and The Washington Times, as well as on WBUR and other outlets. The finding was also featured in the February 13 issue of NIH Research Matters. NIAAA communications staff arranged a post-embargo telebriefing for stakeholders and federal partners, which attracted more than 130 callers. To hear the complete teleconference, visit https://www.youtube.com/watch?v=n1NiekmT828.
Publication Statistics: In the month of March, NIAAA filled orders for 13,643 copies of print publications. As of April 10, there were 30,402 Granicus listserv subscribers to Alcohol Research: Current Reviews; 22,282 to the NIAAA Spectrum; 98 to News Alerts; and 21,841 to receive general information.
Social Media Highlights
NIAAA Twitter: The NIAAA Twitter account (@NIAAAnews) currently has more than 20,600 followers and averages about 100,000 impressions (number of times users saw the tweet on Twitter) per month. NIAAA hosted three Twitter chats in recognition of Alcohol Awareness Month with the National Council on Alcoholism and Drug Abuse, the American Society of Addiction Medicine, and the National Center for Complementary and Integrative Health. The chats covered tips for starting a conversation about treatment with a loved one, resources for friends and families, and alcoh ol treatment resources for clinicians.
ol treatment resources for clinicians.
#NIAAAtraining: In April, NIAAA launched its
#NIAAAtraining series on Twitter to highlight individuals in its postdoctoral and postbaccalaureate training program. The series focuses on the reasons trainees come to work under leading scientists at the Institute and what they gain from the experience.
Seasonal Outreach:
Super Bowl Safety Awareness: NIAAA tweeted Super Bowl safety awareness messages January 30–February 3. Groups and individuals who posted included HHS.gov, Bridgewater State University Outreach Education, and Grand Valley State Unive rsity’s Alcohol Campus Education Services.
rsity’s Alcohol Campus Education Services.
St. Patrick’s Day Safety Awareness:  NIAAA tweeted St. Patrick’s Day safety messages March 15–17. Stakeholder groups were encouraged to disseminate the message on their social media channels. Groups and individuals who posted included Mothers Against Drunk Driving, the American Psychiatric Association, the Indiana Health Department, the Emergency Nurses Association, and the American College Health Association. Overall, the potential audience for the social media outreach effort was about 275,000.
NIAAA tweeted St. Patrick’s Day safety messages March 15–17. Stakeholder groups were encouraged to disseminate the message on their social media channels. Groups and individuals who posted included Mothers Against Drunk Driving, the American Psychiatric Association, the Indiana Health Department, the Emergency Nurses Association, and the American College Health Association. Overall, the potential audience for the social media outreach effort was about 275,000.

Valentine’s Day Health Message:
NIAAA tweeted a Valentine’s Day health message to “Love Your Liver” on February 14, 2018. Groups and individuals who posted included RG Safe Communities and Maryland State Ambassador Lydia Seiders.
Alcohol Awareness Month
In recognition of Alcohol Awareness Month, NIAAA implemented a variety of activities including social media outreach, carousel images, and a Director’s Blog post.
Twitter messages: Throughout the month, @NIAAAnews tweeted informational messages and graphics using the #AlcoholAwarenessMonth hashtag. Tweets included facts about AUD prevalence, treatment options, low-risk drinking limits, health effects,
 and linked to resources like Rethinking Drinking and the NIAAA Alcohol Treatment Navigator.
and linked to resources like Rethinking Drinking and the NIAAA Alcohol Treatment Navigator.
Twitter Chats: NIAAA hosted three Twitter Chats throughout the month. Detailed information can be found above in “Social Media Highlights.”
Carousel Graphics: NIAAA created an Alcohol Awareness Month image carousel for the NIAAA website, linked to Rethinking Drinking. Staff also worked with the NIH Office of the Director to develop a carousel for NIH.gov, linked to the NIAAA Alcohol Treatment Navigator.
NIAAA Director’s Blog: Dr. Koob updated his blog with a new entry recognizing Alcohol Awareness Month. The post can be read here: https://www.niaaa.nih.gov/directors-blog.

Partnerships, Outreach & Public Liaison Activities:
NBC4 Health & Fitness Expo: On March 10 and 11, NIAAA exhibited at the NBC4 Health and Fitness Expo in Washington, D.C. An estimated 85,000 people attend this annual event. NIAAA had two exhibit booths at this year’s expo. One booth offered general information about alcohol, and the other focused on the NIAAA Treatment Navigator, which booth visitors could “test drive” at interactive touchscreen kiosks. Leading up to the event, NIAAA’s Treatment Navigator booth was promoted through banner advertisements on the NBC4 homepage and on their mobile app. These advertisements were both static images and a video, which can be viewed at https://vimeo.com/254748470/02cbbd92d3. In addition, the program booklet for the expo included a quarter-page color advertisement about the NIAAA Treatment Navigator.
Brain Awareness Week: NIH and the National Museum of Health and Medicine in Silver Spring, Maryland, hosted the Museum’s 19th annual Brain Awareness Week on March 14 and 15. Brain Awareness Week, organized by the Dana Alliance for Brain Initiative, is an annual international partnership of government agencies, scientific organizations, and university and volunteer groups dedicated to advancing education about the brain. Various NIH Institutes with neuroscience–related programs offered a series of hands-on demonstrations and exhibits. On March 15, Drs. Mohammed Akbar, Ivana Grakalic, Elizabeth Powell, and Soundar Regunathan ran the Cool Spot Carnival. Starting with a presentation on alcohol and adolescent brain development, students learned about the effect of alcohol on teen/tween brains and the lifelong consequences of underage drinking. Children also had the opportunity to try their hand scoring in a football-toss game while wearing Fatal Vision goggles to simulate being under the influence of alcohol.
U.S.A. Science and Engineering Festival (USASEF): The biennial USASEF was held April 6–8, at the Walter E. Washington Convention Center in Washington, D.C. As part of the NIH Pavilion, NIAAA hosted the Cool Spot Carnival, where participants tossed a ball while wearing Fatal Vision goggles to simulate the effects of alcohol on coordination. The NIH Pavilion had an estimated 50,000+ visitors this year.
Know More Before You Pour: NIAAA and CADCA co-sponsored the Second Annual Know More Before You Pour Challenge. NIAAA provided 10 alcohol-related statistics and invited coalitions to develop social media graphics and marketing plans based on them. Nearly 50 entries were received from coalitions across the United States. Eleven coalitions were recognized for their submissions and selected to receive small stipends to promote the graphic messages. The recognized submissions were displayed at the 2018 CADCA National Leadership Forum, which was held February 6–9 at the Gaylord National Hotel and Convention Center, National Harbor, Maryland.
ScienceBlog.com: In April, NIAAA established a relationship with ScienceBlog.com, which posts press releases and science news stories from individual scientists, research organizations, and universities. ScienceBlog.com has posted three NIAAA Spectrum articles and featured NIAAA content prominently. They will continue to carry NIAAA stories on their homepage and their Our Bloggers section, as well as in their daily email newsletter and on Google News. NIAAA has been given its own blog with links to the NIAAA website, NIAAA Spectrum, and the NIAAA Twitter Feed.
In April, NIAAA established a relationship with ScienceBlog.com, which posts press releases and science news stories from individual scientists, research organizations, and universities. ScienceBlog.com has posted three NIAAA Spectrum articles and featured NIAAA content prominently. They will continue to carry NIAAA stories on their homepage and their Our Bloggers section, as well as in their daily email newsletter and on Google News. NIAAA has been given its own blog with links to the NIAAA website, NIAAA Spectrum, and the NIAAA Twitter Feed.
Additional partner activities:
- The American School Counselors Association hosted a webinar for school counselors on underage drinking on April 18. Dr. Aaron White spoke about “Keeping it Real – and Safe: What Every School Counselor Should Know About Underage Drinking.”
- The spring newsletter of the American Association of Addiction Psychiatry included an article based on NIAAA research, titled “Blackouts: Drowning Memories with Alcohol.”

