NIAAA Director's Report on Institute Activities to the 131st Meeting of the National Advisory Council on Alcohol Abuse and Alcoholism
Table of Contents
Director’s Note
Fall Update – NIAAA’s work combatting the harms of college drinking
As fall begins and students return to college campuses across the country, we once again evaluate our continued efforts to tackle one of the most pervasive issues in higher education ― college drinking and the associated harms.
While scientific progress has been made, changing the culture of risky college drinking has been stubbornly slow. We now have a greater understanding of what works to reduce college drinking and the many problems associated with it; the next hurdle is to make colleges aware of the scientifically supported intervention options available and how best to implement them. I wanted to use this space in the new “Director’s Note” section to highlight some of our recent advances related to this effort.
Effective college drinking interventions are available ― a recent NIAAA-funded study supported the use of targeted strategies developed by campus-community coalitions. This particular intervention, when applied to an average size campus, would result in 228 fewer students experiencing at least one severe consequence of drinking over the course of a month and 107 fewer students injuring others while intoxicated during the year.
Taking such promising interventions into real-world application requires effective collaboration with college administrators. To this end, NIAAA established the College Presidents Working Group in 2011. This group of dedicated college presidents offers input to NIAAA about what information they need and how they would most like to receive it. In response to recent suggestions, NIAAA has engaged top researchers in the field to inform an online “decision support system” to help campuses select evidence-based strategies to meet their individual intervention goals.
Reaching parents is also an important step in helping young adults make good decisions about alcohol. Our seasonal fact sheet, “Fall Semester—A Time for Parents to Discuss the Risks of College Drinking,” was distributed through PR Newswire in August. Just one response: Texas A&M requested more than 500 copies to distribute to parents of incoming freshmen.
We’re also taking some novel approaches to getting the word out. NIAAA has reserved a slot on the David Letterman Times Square Video screen in New York City through the end of September. The video, “Get the Real Picture About College Drinking,” features key college drinking statistics and a link to the NIAAA college webpage.
College drinking is a persistent issue, but as the largest funder of alcohol research, NIAAA is supporting work that will help us make headway in keeping young adults safe during this critical developmental period.
Sincerely,
Ken R. Warren
NIAAA Director
NIAAA BUDGET
FY 2012
The NIAAA is currently closing out FY 2012. In FY 2012 NIH received a total of $30.6 billion after an across the board reduction and the Secretary’s transfer. Major features of the spending bill included the elimination of the National Center for Research Resources (NCRR) and the creation of the National Center for Advancing Translational Sciences (NCATS).
The FY 2012 appropriation for NIAAA provided $459.4 million. This represented a $1.5 million or a 0.3% increase over the FY 2011 comparable level of $457.9 million. NIAAA supported a total of 642 research project grants (RPGs) in FY 2012, including 167 competing awards.
FY 2013
House Action: On July 13th, the House Appropriations Committee released the draft FY 2013 Labor, Health and Human Services (LHHS) funding bill. For NIH, the bill includes $30.6 billion, which is equal to both last year’s level and the President’s request. Within this funding, the legislation includes $175 million for the National Children’s Study, $488 million for Clinical and Translational Sciences Awards, and $376 million for Institutional Development Awards (IDeA) programs. For the NIAAA, the House recommended amount is $459.0 million, a $1.868 million or 0.41% increase over the requested level.
Senate Action: On June 14th the Senate Appropriations Committee approved its FY2013 Labor-HHS spending bill, but has not advanced the bill to the floor. The NIH level proposed by the Senate Committee is $30.6 billion, approximately the same level as the House. For the NIAAA, the Senate recommended amount is $458.5 million, a $1.385 million or 0.3% increase over the requested level.
FY 2014
Preliminary work on the budget for FY 2014 is beginning. After intermediate stages of review, the President’s budget request for FY 2014 will be presented to Congress in February 2013, at which time it will become available to the public.
| FY 2012 Enacted Base* | FY 2013 Requested Level | FY 2013 Senate | FY 2013 House (DRAFT) | |
|---|---|---|---|---|
| Total, NIAAA (In Thousands of Dollars) | 458,972 | 457,104 | 458,489 | 458,972 |
| Change from FY 2012 Enacted | -0.11% | 0.00% | ||
| Change from FY 2013 Requested | 0.30% | 0.41% |
* Enacted base differs slightly from FY 2012 appropriation figure due to transfers, recessions, and comparable adjustments, etc.
DIRECTOR’S ACTIVITIES
| Kenneth R. Warren, Ph.D., Calendar of Activities June – December 2012 |
||
|---|---|---|
| Title of Activity | Date | Director’s Role |
| Research Society of Alcoholism – 35th Scientific Meeting | June 23-27, 2012 | Opening Speaker |
| IOM Neuroscience and Nervous System Disorders Forum | July 11, 2012 | Participant |
| Opening Session of the Consensus Panel (SAMHSA & NIAAA) | July 16-17, 2012 | Opening Speaker |
|
Office of National Drug Control Policy (ONDCP) |
July 25, 2012 | NIAAA Representative |
| Board of Scientific Counselors meeting | August 29-30, 2012 | Participant |
| 7th International Symposium on Alcoholic Liver and Pancreatic Diseases and Cirrhosis, Beijing, China | September 3-8, 2012 | Opening Speaker |
| International Society for Biomedical Research on Alcoholism (ISBRA) 2012 Congress, Sapporo, Japan | September 9/12, 2012 | Plenary Speaker |
| UPCOMING EVENTS | ||
|---|---|---|
| Title of Activity | Date | Director's Role |
| Second European Conference on Fetal Alcohol Spectrum Disorders (FASD), Barcelona, Spain | October 19-25, 2012 | Plenary Speaker |
| Keller Lecture | November 15, 2012 | Host |
| First National Congress of SIFASD (Italian Society for the FASD) | November 19-22, 2012 | Plenary Speaker |
| IOM Neuroscience and Nervous System Disorders Forum | November 28, 2012 | NIAAA Representative |
STAFF TRANSITIONS
 Dr. Lorenzo Leggio has come from Brown University to a joint NIAAA/NIDA appointment as Acting Chief of the Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology in the Laboratory of Clinical and Translational Studies.
Dr. Lorenzo Leggio has come from Brown University to a joint NIAAA/NIDA appointment as Acting Chief of the Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology in the Laboratory of Clinical and Translational Studies.
 Dr. Curtis Carey has come to NIAAA’s Communications and Public Liaison Branch from the National Weather Service, where he was the Director of Communications and Executive Affairs.
Dr. Curtis Carey has come to NIAAA’s Communications and Public Liaison Branch from the National Weather Service, where he was the Director of Communications and Executive Affairs.
 Dr. Sam Zakhari, Director of NIAAA’s Division of Metabolism and Health Effects (DHME), is retiring after more than 25 years of service at the institute. He has accepted a position as Senior Vice President for Science at the Distilled Spirits Council of the United States. His long and distinguished career at NIAAA began in 1986 when he joined us as a Scientific Review Officer, after which he became Director of NIAAA’s Division of Basic Research before transitioning to Director of DMHE.
Dr. Sam Zakhari, Director of NIAAA’s Division of Metabolism and Health Effects (DHME), is retiring after more than 25 years of service at the institute. He has accepted a position as Senior Vice President for Science at the Distilled Spirits Council of the United States. His long and distinguished career at NIAAA began in 1986 when he joined us as a Scientific Review Officer, after which he became Director of NIAAA’s Division of Basic Research before transitioning to Director of DMHE.
STAFF AWARDS
Dr. Joseph Hibbeln, Acting Chief of the Section on Nutritional Neuroscience, won the Wilhelm Normann Metal, the highest award of the Deutsche Gesellschaft für Fettwissenschaft e.V. (German Society for Oils and Lipids Research), to be awarded at the 10th Eur Fed Lipids meeting September 24 in Krakow.
Dr. Pal Pacher, Chief of the Section on Oxidative Stress-Tissue Injury, was awarded tenure by the NIH Central Tenure Committee. Dr. Pacher was also the recipient of the Board of Directors Young Investigator Award and gave a Plenary Lecture at the International Cannabinoid Research Society 2012 Annual Symposium in Freiburg, Germany.
Dr. Mariela Shirley, a program officer in the Division of Epidemiology and Prevention Research, was one of two NIH recipients to be awarded the American Psychological Association’s 2012 Meritorious Research Service Commendation. The award is given to individuals who have made outstanding contributions to psychological science by enhancing opportunities and resources for the field through their service as employees of the federal government or other organizations.
Dr. Yossi Tam, Staff Scientist in the Laboratory of Physiologic Studies, has won an Early Career Development grant-award from The Obesity Society. This is a highly competitive award, given to two individuals annually (out of 87 applications this year). Dr. Tam proposed an independent project to explore the role of soluble leptin receptors and their interactions with the endocannabinoid system in obesity-related leptin resistance.
NIH DIRECTOR’S AWARDS
A number of NIAAA employees were recognized by Dr. Francis Collins with a 2012 NIH Director’s Award for their exceptional contributions in fulfilling the NIH mission.
Dr. Judy Arroyo was recognized for her contributions by the NIH Lesbian, Gay Bisexual and Transgender Research Coordinating Committee.
Dr. Vivian Faden, Maureen Gardner, Shuly Babitz, Jo-Ann Kriebel, and Dr. Trish Powell were recognized for their work on the Alcohol Screening and Brief Intervention for Youth: A Practitioner’s Guide.
Keith Lamirande received the Ruth L. Kirschstein Mentoring Award in recognition of "exemplary performance while demonstrating significant leadership, skill, and ability in serving as a mentor."
Dr. Qi-Ying Liu was honored for his role in the launch of the NIH Blueprint Neurotherapeutics Network (BPN) and contributions to “the exemplary and speedy establishment of a robust ‘virtuapharma’ infrastructure and novel funding mechanism to execute drug development projects through to clinical testing.”
Dr. Peggy Murray received the award for her work on fetal alcohol spectrum disorder research in Russia as part of the Brain Disorders in the Developing World: Research Across the Lifespan program.
Qiaoping Yuan, Basel Baghal, Elisa Moore, and Pei-Hong Shen were recognized for their work in bioinformatics at NIAAA.
NEW RFA’S/PA’S
The NIAAA Division of Metabolism and Health Effects just issued a new program announcement (PA) on stem cell and alcohol research. The title is: “Stem Cells and Alcohol-induced Tissue Injuries" (R01 and R21). (Dr. Peter Gao)
DMHE has also issued PA-12-235 (R01) and PA-12-234 (R21): Unconventional Roles of Ethanol Metabolizing Enzymes, Metabolites, and Cofactors in Health and Disease. (Drs. Andras Orosz and PJ Brooks)
NIAAA has received four proposals in response to PA-11-184 Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grants. (Parent T32)
NIAAA has awarded eight Cooperative Agreement (U01/U24) grants to continue the previously funded “Collaborative Initiative on Fetal Alcohol Spectrum Disorders” (CIFASD), a multidisciplinary consortium of domestic and international projects. The CIFASD aims to accelerate specific areas of research related to the translation of new or improved capabilities in FASD clinical case recognition (through improved diagnosis, enhanced understanding of the domains of neurobehavioral impairment), interventions (nutritional and/or pharmacological) and prevention by fostering collaboration and coordinating basic, clinical, and translational research. Applications from current awardees and new applicants were solicited under RFA-AA-12-004 and RFA-AA-12-005.
NIAAA COMMUNICATIONS & MEDIA COVERAGE
NIAAA HOSTS A RECORD NUMBER OF STUDENTS FROM DEREK JETER’S LEADERSHIP PROGRAM
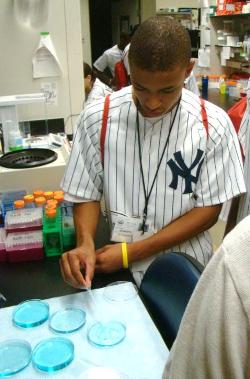 This past July, NIAAA continued its decade-long tradition of hosting high school students participating in baseball star Derek Jeter’s “Jeter’s Leaders” program for a day of presentations, 3D exhibits, and hands-on activities. More than 100 students participated in the trip to NIAAA this year, the largest contingent yet.
This past July, NIAAA continued its decade-long tradition of hosting high school students participating in baseball star Derek Jeter’s “Jeter’s Leaders” program for a day of presentations, 3D exhibits, and hands-on activities. More than 100 students participated in the trip to NIAAA this year, the largest contingent yet.
Events throughout the day mixed alcohol education with information about careers in science. Following an introduction to NIH by Fred Donodeo and a tour of the NIH Clinical Center by Greg Roa, the students met with some of NIAAA’s top scientists. Judy Arroyo wowed the students with a bi-lingual presentation on the history and current state of health science research and people of color, offering encouragement and sage career advice. After a short video presentation on underage drinking, college drinking, and fetal alcohol spectrum disorder (FASD), Dan Falk fielded many questions about treatment and recovery from alcohol problems.
In Fumihito Ono’s Zebrafish lab, the students got a hands-on look at how the small Zebrafish play such a large role in alcohol research and got a taste of life as a post-doc by working with his staff on several interactive activities. NIAAA then invited the students to develop their own short messages about alcohol and adolescence, based on NIAAA information and their own experiences, and present them on camera.
Invited outside scientists brought even more excitement to the day’s activities. Dennis Twombly of the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) led the students through his popular “Drunken Brain” exhibit and slideshow and Archie Fobbs, Curator of the National Museum of Health and Medicine, brought specimens from the world’s largest brain collection. Students screamed in horror and delight as they passed around a preserved human brain while learning about neuroscience and the human nervous system.
In the first ever press release issued by his foundation about the annual visits to NIAAA, Derek Jeter praised the growing relationship, “It is extremely important that our Jeter’s Leaders are properly educated about the negative impact of alcohol abuse and alcoholism. The NIAAA and Turn 2 Foundation collaboration offers our leaders the opportunity to learn about the unhealthy effects of alcohol and strategies to educate peers and younger students.”
Jeter’s Leaders, with programs in New York City and Kalamazoo, MI, fosters leadership and social change, academic achievement, and healthy lifestyles.
ACTIVITIES FOR INTERNATIONAL FETAL ALCOHOL SPECTRUM DISORDERS (FASD) AWARENESS DAY

In September, NIAAA recognized International Fetal Alcohol Spectrum Disorders (FASD) Awareness Day, which takes place each year on September 9. The event serves as a reminder that drinking during pregnancy is the leading preventable cause of birth defects and developmental disabilities in the U.S. Working with Dr. Ken Warren, NIAAA issued a NIH Statement to the Media, which can be viewed at www.niaaa.nih.gov/news-events/news-releases/nih-statement-international-fasd-awareness-day.
“Almost 40 years have passed since we recognized that drinking during pregnancy can result in a wide range of disabilities for children, of which fetal alcohol syndrome (FAS) is the most severe. Yet up to 30 percent of women report drinking alcohol during pregnancy.
…For many years, NIAAA has supported research to understand how alcohol exposure during pregnancy interferes with fetal development and how FASD can be identified and prevented. Scientists continue to make tremendous strides, providing important new insights into the nature of FASD and potential intervention and treatment strategies.
The message is simple, not just on Sept. 9, but every day. Women who are, who may be, or who are trying to become pregnant, should not drink alcohol.”
 Dr. Warren also recorded an in-studio message about International FASD Awareness Day that was posted to the National Organization on Fetal Alcohol Syndrome (NOFAS) website and the NIAAA site.
Dr. Warren also recorded an in-studio message about International FASD Awareness Day that was posted to the National Organization on Fetal Alcohol Syndrome (NOFAS) website and the NIAAA site.
Graphics announcing the day (pictured above) were added to the rotating web displays at NIH.gov and NIAAA.gov. Tweets about the event were also sent out by NIAAA and other institutes.
NIAAA BILLBOARDS ON ALCOHOL AND SUMMER SAFETY

NIAAA “Risky Drinking” billboard on Rt. 1 in Rehoboth, DE, near one of the most popular beaches along the Mid-Atlantic
In July and early August, as a new addition to the seasonal outreach effort, NIAAA ran a series of outdoor ads in select locations across the mid-Atlantic region. The billboards complemented and extended the themes and colorful graphics of the summer safety fact sheets.
The placement strategy called for strategic clusters of ads along the popular roadways leading to Delaware and Maryland beaches, along Interstate 495 in Delaware, and on buses and in Metro stations in the tourist areas of Metropolitan D.C. It also included an aerial billboard running from Ocean City to Rehoboth Beach and an animated 15-second version of the summer safety message on the CBS Times Square/David Letterman billboard in New York City. NIAAA is currently evaluating the audience metrics and other results of these efforts, and these data will inform future decisions about this type of outreach.
PRESS RELEASE
(July 23, 2012) Colleges and communities can reduce alcohol-related harm to students: NIH-supported study finds coordinated efforts limit the impact of high-risk drinking
Coordinated strategies that address alcohol availability, alcohol policy enforcement and drinking norms can help colleges and their communities protect students from the harms of high-risk drinking, according to a new study supported by the National Institutes of Health…
(www.niaaa.nih.gov/news-events/news-releases/colleges-and-communities-can-reduce-alcohol-related-harm)
NEW MULTI-MEDIA PRODUCTS
NIAAA’s Summer Safety Fact Sheet
 For the 2012 summer vacation season, NIAAA continued its seasonal outreach series with its fact sheet, “Don't Let Alcohol Put a Chill on Your Summer Fun.” Fact sheets in the seasonal outreach series contain relevant statistics, practical science-based commentary, and NIAAA website information. They are disseminated through electronic media and in print upon request. The summer safety fact sheet, which was developed with input from the U.S. Coast Guard and National Association of State Boating Law Administrators, focused on the dangers of risky drinking when combined with water recreation and other summer activities.
For the 2012 summer vacation season, NIAAA continued its seasonal outreach series with its fact sheet, “Don't Let Alcohol Put a Chill on Your Summer Fun.” Fact sheets in the seasonal outreach series contain relevant statistics, practical science-based commentary, and NIAAA website information. They are disseminated through electronic media and in print upon request. The summer safety fact sheet, which was developed with input from the U.S. Coast Guard and National Association of State Boating Law Administrators, focused on the dangers of risky drinking when combined with water recreation and other summer activities.
NIAAA distributed the fact sheet to the national media through PR Newswire on May 22 and June 25, 2012, prior to the Labor Day and Independence Day holiday periods, respectively. The release also targeted reporters who have expressed interest in receiving information related to water sports/boating and health. In combination, these efforts generated more than 375 clips on online media outlets, including Yahoo!, the Boston Globe, and the Sacramento Bee, as well as business journals, industry publications, and network television affiliates of FOX, NBC, CBS, and ABC, reaching a total audience of more than 120 million. Additionally, the fact sheet graphic appeared on the Reuters Times Square and PR Newswire Las Vegas Fashion Show Mall billboards.
Alcohol Research: Current Reviews ― Update on the Genetics of Alcoholism
 The Institute’s peer-reviewed journal, formerly Alcohol Research & Health, has a new title, a new design and cover, and new sections, including Resources for Alcohol Use and Abuse Research and a special Letter from the Editor that will introduce each issue. Volume 34, no. 3, 2012 is the first to reflect the new design.
The Institute’s peer-reviewed journal, formerly Alcohol Research & Health, has a new title, a new design and cover, and new sections, including Resources for Alcohol Use and Abuse Research and a special Letter from the Editor that will introduce each issue. Volume 34, no. 3, 2012 is the first to reflect the new design.
NIAAA Spectrum – Issue 10 of the Institute's online webzine, with a focus on college drinking, will be released in September 2012.
The Institute’s popular booklet, Rethinking Drinking, has been translated into Spanish and will be available online and in hard copy for order beginning September 2012.
RECENT NEWS MEDIA INTERVIEWS
Dr. David Goldman, Chief of the NIAAA Laboratory of Neurogenetics, was interviewed by:
- Chelsea Whyte of International Science Times about a study by researchers at Washington University that found a link between alcohol dependency and abnormalities on chromosome 5q13.2. (6/18/12)
Dr. Ralph Hingson, Director, NIAAA Division of Epidemiology and Prevention Research and Dr. Aaron White, Health Science Administrator, DEPR, were interviewed by:
- Andrea McCarren of WUSA-TV in Washington, D.C., for a story about the impact of alcohol consumption on a developing adolescent brain. The story will air in early September. (8/16/12)
Dr. Hingson was also interviewed by:
- Bruce Schreiner of the Associated Press, for a story about college drinking prevention programs. (8/7/12)
- Kevin Kertscher, of the Dartmouth Center for Health Science Studies, for a web-based video series about college drinking and the Dartmouth-led National College Health Improvement Project. (7/10/12)
Dr. George Kunos, NIAAA Scientific Director, was interviewed by:
- Rachael Rettner of MyHealthNewsDaily.com, to discuss a study recently reported by Dr. Kunos and colleagues in Cell Metabolism that found that certain molecules (peripherally restricted CB1R inverse agonists) have therapeutic potential in obesity. (7/25/12)
- Anette Breindl of BioWorld Today, to discuss the Cell Metabolism paper by Dr. Kunos and colleagues. (7/30/12)
Dr. Raye Litten, Associate Director, NIAAA Division of Treatment and Recovery Research, was interviewed by:
- Thomas Betar of the Deseret News in Salt Lake City, regarding the latest treatments for alcohol dependence. (6/6/12)
- Lissy de la Rosa of Siempre Mujer magazine, published in Spanish and distributed in the U.S. and the Caribbean, regarding treatments for alcoholism. (8/15/12)
Dr. Gary Murray, Program Director, NIAAA Division of Metabolism and Health Effects, was interviewed by:
- Meredith Melnick of the Huffington Post, regarding the effectiveness of hangover treatments. (9/6/12)
- Lettie Teague, wine columnist for the The Wall Street Journal, to discuss recent research on the health effects of wine. (9/12/12)
Dr. White was also interviewed by:
- Evan Bleier of Maxim magazine, to respond to a reader-submitted question about why some people continue to drink after they have become drunk. (8/22/12)
- Gabriele Jimenez of Revista VEJA, Brazil’s bestselling weekly news publication, to discuss alcohol poisoning, blackouts, and other dangerous effects of alcohol on teenagers. (7/5/12)
- Rachel Morse of Student Health 101, an online monthly health magazine for college students, to discuss the dangerous effects of risky drinking among college students. (7/11/12)
- Tim Sheehan of the Fresno Bee, about rates of binge drinking and alcohol poisoning on college campuses. (9/7/12)
Dr. Sam Zakhari, Director of the NIAAA Division of Metabolism and Health Effects, gave interviews on the health effects of alcohol to:
- Susan Matthews of MyHealthNewsDaily.com. (8/13/12)
- Harriet Matthews, of National Geographic (UK). (8/13/12)
- Kimberly Goad of Consumer Reports' Shopsmart magazine. (8/15/12)
- Hal B. Klein, a freelance radio producer working on a story for National Public Radio. (8/6/12)
- Jeremy Balch, medical editor for Hepatology Digest. (9/7/12)
Select quotes in the press (organized by date)
…According to Dr. Aaron White, Program Director for Underage and College Drinking Prevention Research at the National Institute of Alcohol Abuse and Alcoholism (NIAAA), “It can be quite difficult for an outside observer to tell if someone is in a blackout. The person could seem aware and articulate, but without any memory being recorded.”… The way college students drink increases the odds of blackouts, says Dr. White. “Alcohol is more likely to cause a blackout when it gets into your body, and therefore your brain, fast. It catches the memory circuits off guard and shuts them down. Doing shots or chugging beer, and doing it on an empty stomach, gets the alcohol into your bloodstream quickly.”…
(“New Studies Shed Much-Needed Light on Alcohol-Induced Memory Blackouts,” Join Together, June 15, 2012)
"Alcoholism's pervasive impact on public health and its heritability make searches for genes influencing vulnerability a priority," said David Goldman, chief of the lab of neurogenetics at the National Institute on Alcohol Abuse and Alcoholism. "Although only a few genes influencing alcoholism risk have been discovered so far, we can expect this picture to change rapidly as more powerful genomic tools, including genotyping arrays and next-generation sequencing, are applied, and as geneticists become ever more ambitious in the size and phenotypic depth of the populations they study."
(“Genes May Determine Alcohol Dependence,” International Science Times, June 18, 2012)
…Today, 18 million Americans still suffer from alcohol-use disorder and 76 million adults suffer worldwide from the condition, said Dr. Raye Litten, health scientist administrator in the National Institute on Alcohol Abuse and Alcoholism's Division of Treatment and Recovery Research…"We have been investigating new medications that give patients more of a menu to choose from," he said. "If one doesn't work then they try another. Alcoholism is a serious problem. The more weapons you have and the more ways you can treat it, the better."…
(“Evolving alcohol treatments can work wonders,” Deseret News [Salt Lake City], July 24, 2012)
Now the trick is to spread those proven strategies to more campuses, said Dr. Ralph Hingson, director of NIAAA's Division of Epidemiology and Prevention Research. "Even though our knowledge about how to reduce these problems is increasing, on a national basis we don't see much change," he said. "So the challenge is how to persuade more colleges and universities to take these approaches that have been found to be effective in scientific studies and apply those interventions, not cook up their own interventions.”
(“UK, UofL Team Up To Curb Alcohol Abuse,” Associated Press, August 7, 2012)
"If parents have a liberal idea about alcohol, kids may get the wrong message," says Dr. Vivian Faden, director of the Office of Science Policy and Communications at the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health. "Underage drinking can lead to injuries, fatal car accidents, risky sexual behavior, and there's also potential risk to the developing brain."
(“Is underage drinking ever OK?,” CNN, September 2, 2012)
SELECT NIAAA STAFF ACTIVITIES
MARQUIS ACTIVITIES (ORGANIZED BY DATE)
Dr. Judith Arroyo attended the 24th Annual Native Health Research Conference held July 16-19 in Seattle, WA, which featured presentations from several NIAAA-funded investigators. Dr. Arroyo presented an invited plenary lecture on “Advances in Alcohol Research that Promote Indigenous Health.”
Dr. Kendall Bryant, NIAAA Alcohol and AIDS Coordinator, attended the 2012 International AIDS conference held July 22-27 in Washington D.C. where he presented Alcohol and HIV/AIDS research goals at the NIH-organized grantsmanship workshop and as an NIH expert and facilitator during special open sessions for researchers and attendees. The conference is the largest international meeting on HIV representing all the stakeholders in the global response to the AIDS epidemic with the critical theme of “Turning the Tide.”
Drs. Ellen Witt and Ivana Grakalic of the Division of Neuroscience and Behavior served as co-organizers and session moderators for the NIH Basic Behavioral and Social Science Opportunity Network Workshop: “Improving Animal Models of Human Behavioral and Social Processes” on July 23-24 in Rockville, MD. This workshop brought together experts from the scientific community with the goals 1) to identify animal models of human behaviors that have been particularly successful and generative, so that we can learn from those successes to guide future activities, and 2) to discuss strategies for improving animal models of human behavioral and social processes that have been particularly challenging to model in animals.
Dr. Ralph Hingson presented “What Works to Reduce Youth Drinking?” at the first meeting of the World Health Organization/Pan American Health Organization (WHO/PAHO) on August 22 in Mexico City. Dr. Hingson also spoke before the Office of National Drug Control Policy on “Preventing Drug-Impaired Driving and Overdoses: Lessons from Alcohol-Impaired Driving” on July 26, 2012 in Washington, D.C.
Dr. Sam Zakhari, Director of the Division of Metabolism and Health Effects (DMHE), gave a presentation titled “The Bermuda Triangle for the Liver: Alcohol, Obesity, and Viral Hepatitis” at the 7th International Symposium on Alcoholic Liver and Pancreatic Diseases (ALPD) and Cirrhosis on September 6 in Beijing, China.
Dr. Witt attended Unveiling the NIH Toolbox Conference, held September 10-11, which gave an overview of the development, testing, and use of an integrated set of tools for measuring cognitive, emotional, motor and sensory function in biomedical research. These tools were validated for use in diverse cultures, ethnic and geographic groups, ages (3-85 years) and study types. The toolbox is expected to provide a more complete picture of neurological and behavioral health in large-scale longitudinal studies, epidemiological studies, and clinical trials; and to facilitate cross-study comparisons. Dr. Ellen Witt was the NIAAA representative to the NIH project team that provided oversight to this project.
The 35th Annual Scientific Meeting of the Research Society on Alcoholism was held June 23-27 in San Francisco, CA. Below is a description of contributions by NIAAA staff members. (Organized alphabetically)
Dr. Judith Arroyo, NIAAA Minority Health and Health Disparities (MHHD) Coordinator, served as discussant on one MHHD-related RSA symposia and chaired another. These two well-attended sessions were a marked improvement over the past few years, when no MHHD-related symposia were on the agenda. NIAAA hopes to use this as a means of initiating a MHHD research interest group at RSA.
Dr. Roz Breslow, Division of Epidemiology and Prevention Research (DEPR), presented original research titled “Diets of Drinkers on Drinking and Non-drinking Days” at RSA in an NIAAA-sponsored symposium titled “Does Alcohol Affect Healthy Eating and Weight Control?”
Dr. Bob Freeman, DEPR, organized and co-chaired the symposium “Investigating the Alcohol Use-Sexual Risk Behavior Relationship Using Survey, Experimental, and Neurocognitive Research Methods.”
Dr. Ivana Grakalic, Division of Neuroscience and Behavior (DNB), organized a symposium entitled “Does Alcohol Affect Healthy Eating and Weight Control?” This symposium presented recent research on the relationship between alcohol drinking and healthy eating and weight control with emphasis on psychological and physiological variables that control eating and satiety. Dr. Grakalic also gave an overview of the state of science as an introduction to the symposium.
Dr. Ralph Hingson, Director, DEPR, presented a talk entitled “Adolescents at Risk for Excess Alcohol Consumption are Infrequently Asked or Counseled about Drinking Alcohol.”
Dr. Robert Huebner, Director of the Division of Treatment and Recovery Research (DTRR), provided an update on NIAAA research programs and funding opportunities to the 8th Annual RSA Satellite Session on Mechanisms of Behavior Change on June 29 at RSA. He also served on the planning committee for this annual one-day conference. The satellite session drew over 120 researchers.
Dr. Kathy Jung, Division of Metabolism and Health Effects (DMHE), organized a session with Dr. Laura Nagy entitled, “Interactions of Immune and Non-immune Signaling in Alcoholic Liver Disease.”
Dr. Raye Litten, DTRR, presented “What Have We Learned about Alcohol Clinical Trial Methodology? New Approaches for Determining Treatment Endpoints,” and presented “Clinical Trials and Certificates of Confidentiality.” He also organized a symposium with Dr. Huebner entitled “Is Abstinence the Only Endpoint? Advances in Alternative Treatment Endpoints Across Populations and Treatment Setting.”
Dr. Antonio Noronha, Director, DNB, gave an introduction to a symposium entitled “The Relationship Between Epigenetic Modifications, Gene Expression, and Alcohol,” which highlighted recent progress in the study of the effects of alcohol on epigenetic processes including chromatin remodeling, DNA methylation, and transposable element silencing.
Dr. Noronha also organized, chaired and gave the introductory presentation at a symposium entitled “Neurobiological and Behavioral Correlates of Adolescent Alcohol Exposure at Adulthood.” This symposium highlighted recent findings from this major national consortium funded by NIAAA on the effects of adolescent alcohol exposure on brain development and behavior in adulthood.
Dr. Andras Orosz, DMHE, organized a symposium for the RSA: “Proteostasis (Protein Homeostasis): A Novel Mechanism in Alcohol Addiction and Alcohol-Induced End Organ Injury.” Dr. Orosz also organized a new trans-NIH Scientific Interest Group (SIG) “Proteostasis.”
Dr. Soundar Regunathan, DNB, chaired a symposium entitled “Moderate Ethanol Ingestion on Cardio-Cerebral Vascular Function, Inflammation and Stroke.” He also served as an introductory speaker and discussant.
Dr. Matthew Reilly, DNB, co-chaired and served as a discussant at the symposium “Molecular Mechanisms of Alcohol-Induced Fetal Programming.” This symposium highlighted new molecular findings that contribute to fetal programming after in utero alcohol exposure with an emphasis on epigenetic mechanisms. Dr. Reilly focused the discussion on the mechanisms that produce long-term, stable alcohol-induced epigenetic modifications leading to adult disease as well as the genetic factors that might also contribute to these stable changes. \
Dr. Megan Ryan, DTRR, presented a talk on “Clinical Trials and Certificates of Confidentiality,” and presented the poster “Measuring Average Alcohol Consumption: Comparison of a Brief Drinking Questionnaire to an Abbreviated Version of the Form 90.”
Dr. Joe Wang, DMHE, co-organized and co-chaired a symposium entitled: “Alcohol-HCV Related Liver Disease: Newly Recognized Ethanol Effects Controlling Lipid Metabolism, Viral Replication and Antiviral Signaling.”
The 2012 International Society for Biomedical Research on Alcoholism (ISBRA) World Congress took place on September 9-12 in Sapporo, Japan.
Dr. Patricia Chou, Mathematical Statistician (Biomedical) in the Laboratory of Epidemiology and Biometry (LEB) was an invited speaker/presenter with former NIAAA Director Dr. T.K. Li. Together they co-organized, co-chaired, and presented at a symposium entitled “Alcohol-Related Injury: Evidence from International Emergency Department Studies, with Special Focus on Asia.”
Dr. Bin Gao, Chief of the Laboratory of Liver Diseases (LLD), was an invited speaker and co-chair of a session on the “LLD’s Important Research Findings on the Therapeutic Potentials of Interleukin-22 for the Treatment of Alcoholic Liver Disease” – which is critical to promote translation research and clinical trials using interleukin-22 for treatment of this disease.
Dr. Andrew Holmes, Chief of the Laboratory of Behavioral and Genomic Neuroscience (LBGN), served as an organizer, chair and speaker for the symposium, “Alcohol Effects on Prefrontal Function,” which includes renowned alcohol experts from the US, Europe and Japan, to present research examining the effects of alcohol on cognitive functions that are impaired in alcoholism.
Dr. Hiromi Ikeda, Research Fellow in the Laboratory of Molecular Physiology (LMP) was invited to present data on zebrafish models, developed by the LMP, which they are using to study the mechanism of neuronal intoxication. LMP data suggest that nicotinic acetylcholine receptors contribute to the development of acute tolerance to ethanol. This is an important finding in the field of alcohol research from both a scientific and methodological point of view.
David Lovinger, Chief of the Laboratory of Integrative Neuroscience (LIN), organized, chaired and presented at the symposium entitled “Influence of Development and Developmental Stress on Excessive Alcohol Intake in Rodents and Non-Human Primates.”
Dr. Sam Zakhari, Director, DMHE, co-chaired two symposia on “Alcohol and Cancer” and “Role of Cytokines and Innate Immune Signaling in Alcoholic Liver Disease: Progression from Steatohepatitis to Fibrosis and Cancer.” Dr. Zakhari also gave a presentation on “Alcohol and Cancer: What and Why?” and an overview talk for a symposium entitled “Second and Multiple Hits in Alcohol Liver Disease” on September 9, 2012.
Dr. Zakhari delivered a presentation titled “Molecular Mechanisms of Alcohol-Induced Liver Injury” at the ISBRA Satellite meeting in Kyoto, Japan on September 14.
OTHER STAFF ACTIVITIES
Division of Epidemiology and Prevention Research (DEPR)
Dr. Hingson, Division Director, presented at the Community Anti-Drug Coalitions of America’s (CADCA) 2012 Mid-Year Training Institute in Nashville, TN, on the topic of “New Research since the Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking.” In May, he addressed the Society for Prevention Research Annual Meeting in Washington, D.C., presenting on “Preventing Drug-Impaired Driving: Lessons from Alcohol-Impaired Driving.”
Dr. Bob Freeman presented “Women’s Health Research at the National Institute on Alcohol Abuse and Alcoholism” as part of a panel on Women’s Health Research at the NIH at the 5th National Conference on Behavioral Health for Women and Girls: Health, Empowerment, Resilience and Recovery.
Dr. Freeman represented NIAAA at a “Meet the Experts” session of the 2012 NIH Regional Seminar on Program Funding and Grants Administration and served as a member of the Conference Planning Committee and Chair of the Efficacy Research Programmatic Track for the Society for Prevention Research’s 20th Annual Meeting. He also served as a panelist for the meeting, speaking about “Research on the Prevention of Bullying.”
Dr. Mariela Shirley co-chaired a symposium on “Stimulant Medication, ADHD, and Substance Use Outcomes” and a roundtable discussion panel entitled “An Insiders’ Guide to NIH Research and Training Opportunities: Talk with NIH Staff” at the annual meeting of the American Psychological Association, held in Orlando, FL.
Dr. Shirley also took part in a webinar on early career research funding opportunities for behavioral scientists that was presented to the American Psychological Association, Psychological Study of Ethnic Minority Issues (Division 45) in May.
Division of Neuroscience and Behavior (DNB)
Drs. Antonio Noronha, Division Director, and Matthew Reilly participated in the steering committee meeting for the NIAAA funded consortium entitled “Mechanisms of Alcohol Pathology: A Collaborative Partnership Between NCCU & UNC” in San Francisco, CA on June 25, 2012. This consortium is a major part of the Institute’s portfolio in Minority Health & Health Disparities Research. Progress on all three center projects was presented as well as preliminary data for newly funded pilot projects. Future directions include projects aimed at examining methionine metabolizing enzymes in alcohol-induced liver injury, characterization of ethanol-induced Shh variants in fetal alcohol spectrum defects, and the effects of in utero alcohol exposure on mammary tumor risk.
Drs. Lindsey Grandison and John Matochik participated in the “Meet the Experts 1:1” sessions at the 2012 NIH Regional Seminar on Program Funding and Grants Administration held in Washington, DC. The NIH Regional Seminar is held throughout the year at different locations to provide grant applicants and staff from institutional sponsored research offices with information about NIH policies and procedures. The “Meet the Experts 1:1” session provided the opportunity for attendees to meet on an individual bases with NIH staff to ask questions and seek advice.
Dr. John Matochik helped to organize a workshop on “The NIH Role in Neuroimage Data Sharing” that was sponsored by the NIH Blueprint for Neuroscience Research. The purpose of the two-day workshop was to solicit advice and comments for the development of policies related to sharing on imaging data from NIH-supported studies.
NIAAA Minority Health and Health Disparities (MHHD)
Dr. Judith Arroyo, NIAAA Minority Health and Health Disparities (MHHD) Coordinator, along with staff rom NCI, conducted Technical Assistance Workshops as part of outreach efforts to encourage applications on PAR-11-346 Interventions for health promotion and disease prevention in Native American populations. One workshop was presented in Anchorage, Alaska on August 2nd in conjunction with the annual meetings of the Association of American Indian Physicians. Another was held in Fairbanks, Alaska in conjunction with the Circumpolar Research Meeting.
For staff activities from Division of Treatment and Recover Research (DTRR) and Division of Metabolism and Health Effects (DMHE), see the previous sections on the Scientific Meeting of the Research Society on Alcoholism and the International Society for Biomedical Research on Alcoholism (ISBRA) World Congress.
NEW AND UPCOMING PUBLICATIONS/MULTI-MEDIA PRODUCTS
 Changhai Cui, Lindsey Grandison, and Antonio Noronha are editors for the book on “Neural-Immune Interactions in Brain Function and Alcohol Related Disorders” (ISBN 978-1-4614-4728-3, Springer, 2012, http://www.springer.com/biomed/neuroscience/book/978-1-4614-4728-3).
Changhai Cui, Lindsey Grandison, and Antonio Noronha are editors for the book on “Neural-Immune Interactions in Brain Function and Alcohol Related Disorders” (ISBN 978-1-4614-4728-3, Springer, 2012, http://www.springer.com/biomed/neuroscience/book/978-1-4614-4728-3).
This book integrates emerging knowledge on neural-immune interactions with related key discoveries in alcohol research to provide a comprehensive overview of neuroimmune modulation of brain function and behavior associated with alcohol use disorders. It highlights novel roles of neuroimmune factors in brain function and development, as well as in CNS dysfunctions, including neuroAIDS, neuropsychiatric disorders, and addiction.
Drs. Marcia Scott and Judith Arroyo served as guest editors on a special edition of the American Journal American Journal of Drug and Alcohol Abuse in press. This special edition will feature articles demonstrating the state of the science in American Indian/Alaska Native research, including several NIAAA funded investigators. This will be the first known journal edition including only data-based articles on AIAN research on drug and alcohol abuse.
Coinciding with the move to the electronic submission of applications for Diversity Supplement (DS), the MHHD group has developed a new NIAAA website for extramural investigators on the NIAAA process and procedures for Diversity Supplements (http://www.niaaa.nih.gov/grant-funding/funding-opportunities/diversity-supplements).
Review and Editorial Articles:
Dr. Matthew Reilly served as editor of a section on Non-coding RNAs and Addiction in a special issue of Frontiers in Genetics. His editorial focuses on the emerging role of non-coding RNA in alcohol neuroadaption and its implications for understanding and treating alcoholism and related disorders.
Reilly MT. Role of non-coding RNAs in the neuroadaptation to alcoholism and fetal alcohol exposure. Frontiers in Genetics, 2012 Jun 15.
Dr. Reilly and Dr. Antonio Noronha wrote an article for the August issue of Alcohol Research: Current Reviews describing the current and future use of animal models to understand the genetic basis of alcoholism.
Reilly MT, Harris RA, Noronha A. Using genetically engineered animal models in the post-genomic era to study gene function in alcoholism. Alcohol Research: Current Reviews, 2012 Aug; 34(3): 282-291 (Epub ahead of print).
Drs. Joe Wang and Sam Zakhari authored a review article summarizing recent advances in understanding the origins and roles of various inflammatory components in alcoholic liver disease.
Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annual Review of Nutrition. 2012 Aug 21;32:343-68.
WHAT’S AHEAD?
October
Dr. Soundar Regunathan will be organizing a satellite symposium entitled “Neural mechanisms of Pain and Alcohol Dependence” at the Society for Neuroscience Annual Meeting in New Orleans on October 13, 2012. This symposium will discuss the current status of research on neurobiological mechanisms influencing pain and alcohol use. The featured speakers include leading NIAAA- funded alcohol investigators, George Koob and Jon Levine, and leading pain investigators, Vania Apkarian and Volker Neugebauer.
Drs. Lindsey Grandison and John Matochik will be co-chairs of a symposium at the annual meeting of the Society for Neuroscience in New Orleans. The symposium titled “Prefrontal Cortex-Amygdala Interactions in Control of Behavior” will feature investigators from both Europe and the United States (including two NIAAA-supported grantees) describing their cutting-edge advances in this area.
Dr. Dionne Godette will present a poster on “Ethnic vs. Racial Determinants of Alcohol-related Problems: Disparities at the Intersection” at the American Public Health Association’s 140th Annual Meeting in San Francisco, CA. The poster will be presented during the session on Race, Ethnicity and Special Populations: Epidemiology and Interventions, scheduled for Tuesday, October 30, 2012 from 2:30 p.m. -3:30 p.m.
November
NIAAA will host the annual Keller Honorary Lecture on November 15, 2012, in Lipsett auditorium in Bethesda, MD. The honoree, Dr. Kenneth Kendler, will present “Genetic Epidemiology of Alcohol Use Disorders: Current Perspectives.” Dr. Kendler is the Director of Virginia Commonwealth University’s Institute for Psychiatric and Behavioral Genetics and the Rachel Brown Banks Distinguished Professor of Psychiatry and Professor of Human Genetics. He is also the Director of the Psychiatric Genetics Program at VCU and co-Director of the VCU Alcohol Research Center. Dr. Kendler is a world-renowned expert on the genetics of psychiatric and substance abuse disorders.
December
Drs. Dionne Godette and Mariela Shirley are serving as planning committee members for the NIAAA meeting, “Establishing a Taxonomy of Alcohol-Related Environmental Exposures on Associated Alcohol Use and Consequences workshop,” tentatively schedules for December 6 - 7, 2012 in Rockville, Maryland.
NIAAA RESEARCH HIGHLIGHTS
Intramural Research
A Double-Blind, Placebo-Controlled Trial Assessing the Efficacy of Levetiracetam Extended-Release in Very Heavy Drinking Alcohol-Dependent Patients
The anticonvulsant levetiracetam(sold under the brand-name Keppra) was not shown to be effective in treating alcohol-dependent patients in a multisite clinical trial. Previous studies had suggested that the drug might hold promise in reducing drinking but a randomized controlled trial showed no difference in alcohol consumption between patients receiving levetiracetam extended release and a placebo.
Despite advances in the development of medications to treat alcohol dependence, few medications have been approved by the U.S. Food and Drug Administration. The use of certain anticonvulsant medications has demonstrated potential efficacy in treating alcohol dependence. Previous research suggests that the anticonvulsant levetiracetam may be beneficial in an alcohol-dependent population of very heavy drinkers. In this double-blind, randomized, placebo-controlled clinical trial, 130 alcohol-dependent patients who reported very heavy drinking were recruited across 5 clinical sites. Patients received either levetiracetam extended-release (XR) or placebo and a Brief Behavioral Compliance Enhancement Treatment intervention. Levetiracetam XR was titrated during the first 4 weeks to 2,000 mg/d. This target dose was maintained during weeks 5 through 14 and was tapered during weeks 15 and 16. No significant differences were detected between the levetiracetam XR and placebo groups in either the primary outcomes (percent heavy drinking days and percent subjects with no heavy drinking days) or in other secondary drinking outcomes. Treatment groups did not differ on a number of nondrinking outcomes, including depression, anxiety, mood, and quality of life. The only difference observed was in alcohol-related consequences. The levetiracetam XR treatment group showed significantly fewer consequences than did the placebo group during the maintenance period (p = 0.02). Levetiracetam XR was well tolerated, with fatigue being the only significantly elevated adverse event, compared with placebo (53% vs. 24%, respectively; p = 0.001). This multisite clinical trial showed no efficacy for levetiracetam XR compared with placebo in reducing alcohol consumption in heavy drinking alcohol-dependent patients. (Fertig JB, et al. Alcoholism: Clinical and Experimental Research, 2012 Aug;36(8):1421-1430)
Chronic alcohol remodels prefrontal neurons and disrupts NMDA receptor-mediated fear extinction encoding
The study suggests that chronic alcohol use may increase the risk for post-traumatic stress disorder (PTSD) by altering the brain’s ability to recover from a traumatic experience. While alcoholism is often linked with PTSD, few studies have explored how chronic drinking may subsequently make a person more prone to such anxiety disorders. In a new study, researchers supported by NIAAA observed that chronic alcohol exposure altered neurons in the medial prefrontal cortex (mPFC) of mice, making them slower to suppress a conditioned fear response.
Alcoholism is frequently co-morbid with posttraumatic stress disorder (PTSD) but it is unclear how alcohol impacts neural circuits mediating recovery from trauma. We found that chronic intermittent ethanol (CIE) impaired fear extinction and remodeled the dendritic arbor of medial prefrontal cortical (mPFC) neurons in mice. CIE impaired extinction encoding by infralimbic (IL) mPFC neurons in vivo, and functionally downregulated burst-mediating NMDA GluN1 receptors. These findings suggest alcohol may increase risk for trauma-related anxiety disorders by disrupting mPFC-mediated extinction of fear. (Holmes A, et al., Nature Neuroscience, 2012 Sept 2 [Epub ahead of print])
Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity
New findings by NIAAA scientists suggest that boosting the level of anandamide, a natural brain chemical similar to marijuana, may help people cope with stress. In a series of experiments with mice, researchers showed that augmenting and prolonging the effect of anandamide in a brain region called the amygdala reduced the animals’ fear and stress responses. They then showed that humans who have a gene that makes a reduced amount of the enzyme that breaks down anandamide also have lower scores on tests that measure stress.
Endocannabinoids are released ‘on-demand’ on the basis of physiological need, and can be pharmacologically augmented by inhibiting their catabolic degradation. The endocannabinoid anandamide is degraded by the catabolic enzyme fatty acid amide hydrolase (FAAH). Anandamide is implicated in the mediation of fear behaviors, including fear extinction, suggesting that selectively elevating brain anandamide could modulate plastic changes in fear. Here we first tested this hypothesis with preclinical experiments employing a novel, potent and selective FAAH inhibitor, AM3506 (5-(4-hydroxyphenyl)pentanesulfonyl fluoride). Systemic AM3506 administration before extinction decreased fear during a retrieval test in a mouse model of impaired extinction. AM3506 had no effects on fear in the absence of extinction training, or on various non-fear-related measures. Anandamide levels in the basolateral amygdala were increased by extinction training and augmented by systemic AM3506, whereas application of AM3506 to amygdala slices promoted long-term depression of inhibitory transmission, a form of synaptic plasticity linked to extinction. Further supporting the amygdala as effect-locus, the fear-reducing effects of systemic AM3506 were blocked by intra-amygdala infusion of a CB1 receptor antagonist and were fully recapitulated by intra-amygdala infusion of AM3506. On the basis of these preclinical findings, we hypothesized that variation in the human FAAH gene would predict individual differences in amygdala threat-processing and stress-coping traits. Consistent with this, carriers of a low-expressing FAAH variant (385A allele; rs324420) exhibited quicker habituation of amygdala reactivity to threat, and had lower scores on the personality trait of stress-reactivity. Our findings show that augmenting amygdala anandamide enables extinction-driven reductions in fear in mouse and may promote stress-coping in humans. (Gunduz-Cinar O, et al., Molecular Psychiatry, 2012 June 12 [Epub ahead of print])
Interleukin 22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway
Alcohol use is a leading cause for pancreatitis, an inflammation of the pancreas caused when digestive enzymes are activated before being secreted into the small intestine. Pancreatitis can be fatal and currently, there is no specific treatment for the disease. This study suggests that interleukin-22, a cytokine involved in cellular inflammatory response, holds promise for developing a drug treatment.
Pancreatitis occurs when digestive enzymes are activated in the pancreas. Severe pancreatitis has a 10-30% mortality rate. No specific treatments for pancreatitis exist now. Here, we discovered that interleukin-22 (IL-22) may have therapeutic potential in treating acute and chronic pancreatitis. Wild-type and IL-22 knockout mice were equally susceptible to cerulein-induced acute and chronic pancreatitis, whereas liver-specific IL-22 transgenic mice were completely resistant to cerulein-induced elevation of serum digestive enzymes, pancreatic necrosis and apoptosis, and inflammatory cell infiltration. Treatment of wild-type mice with recombinant IL-22 or adenovirus IL-22 markedly attenuated the severity of cerulein-induced acute and chronic pancreatitis. Mechanistically, we show that the protective effect of IL-22 on pancreatitis was mediated via the induction of Bcl-2 and Bcl-X(L), which bind to Beclin-1 and subsequently inhibit autophagosome formation to ameliorate pancreatitis. In conclusion, IL-22 ameliorates cerulein-induced pancreatitis by inhibiting the autophagic pathway. IL-22 could be a promising therapeutic drug to treat pancreatitis. (Feng D, et al., International Journal of Biological Sciences, 2012;8(2):249-57)
Peripheral Cannabinoid-1 Receptor Inverse Agonism Reduces Obesity by Reversing Leptin Resistance
Research shows that an experimental drug that targets molecular sites in the liver and other tissues without acting on identical sites in the brain holds promise as an effective treatment for obesity and its complications. The current findings are part of ongoing studies on the endocannabinoid system, which regulates appetite and the metabolism of lipids, and is therefore implicated in obesity, diabetes, alcoholism and cardiovascular disease.
Obesity-related leptin resistance manifests in loss of leptin’s ability to reduce appetite and increase energy expenditure. Obesity is also associated with increased activity of the endocannabinoid system, and CB1 receptor (CB1R) inverse agonists reduce body weight and the associated metabolic complications, although adverse neuropsychiatric effects halted their therapeutic development. Here we show that in mice with diet-induced obesity (DIO), the peripherally restricted CB1R inverse agonist JD5037 is equieffective with its brain-penetrant parent compound in reducing appetite, body weight, hepatic steatosis, and insulin resistance, even though it does not occupy central CB1R or induce related behaviors. Appetite and weight reduction by JD5037 are mediated by resensitizing DIO mice to endogenous leptin through reversing the hyperleptinemia by decreasing leptin expression and secretion by adipocytes and increasing leptin clearance via the kidney. Thus, inverse agonism at peripheral CB1R not only improves cardiometabolic risk in obesity but has antiobesity effects by reversing leptin resistance. (Joseph T, et al., Cell Metabolism, 2012 July 25 [Epub ahead of print])
Extramural Research
A Randomized Controlled Trial of Event-Specific Prevention Strategies for Reducing Problematic Drinking Associated With 21st Birthday Celebrations.
This study demonstrated the effectiveness of several intervention approaches in decreasing the amount of alcohol students drink while celebrating their 21st birthday and minimizing resulting harms. Brief Alcohol Screening and Intervention of College Students (BASICS) was most effective when delivered in person but also effective when administered online. An Event Specific Prevention approach was equally effective when administered online as in person.
While research has documented heavy drinking practices and associated negative consequences of college students turning 21, few studies have examined prevention efforts aimed at reducing high-risk drinking during 21st birthday celebrations. The present study evaluated the comparative efficacy of a general prevention effort (i.e., Brief Alcohol Screening and Intervention for College Students, or BASICS) and event-specific prevention in reducing 21st birthday drinking and related negative consequences. Furthermore, this study evaluated inclusion of peers in interventions and mode of intervention delivery (i.e., in-person vs. via the Web). Method: Participants included 599 college students (46% male): men who intended to consume at least 5 drinks and women who intended to consume at least 4 drinks on their 21st birthday. After completing a screening/baseline assessment approximately 1 week before turning 21, participants were randomly assigned to 1 of 6 conditions: 21st birthday in-person BASICS, 21st birthday web BASICS, 21st birthday in-person BASICS plus friend intervention, 21st birthday web BASICS plus friend intervention, BASICS, or an attention control. A follow-up assessment was completed approximately 1 week after students' birthdays. Results: Results indicated a significant intervention effect for BASICS in reducing blood alcohol content reached and number of negative consequences experienced. All 3 in-person interventions reduced negative consequences experienced. Results for the web-based interventions varied by drinking outcome and whether a friend was included. Conclusions: Overall, results provide support for both general intervention and ESP approaches across modalities for reducing extreme drinking and negative consequences associated with turning 21. These results suggest there are several promising options for campuses seeking to reduce both use and negative consequences associated with 21st birthday celebrations. (Neighbors C, et al. Journal of Consulting and Clinical Psychology, 2012 Jul 23. [Epub ahead of print])
Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats
NIAAA scientists recently reported that the brain’s glucocorticoid system appears to be involved in the development of alcohol dependence and may represent a potential target for treating alcoholism. Experiments with rats showed that animals that became alcohol dependent and then went through alcohol withdrawal had fewer glucocorticoid receptors in brain areas involved in stress and reward, but that the receptors became replenished with prolonged alcohol abstinence. Researchers also showed that a compound that blocks glucocorticoid receptors prevented rats from escalating their alcohol intake after their initial exposure to alcohol vapor.
Rats exposed to alcohol vapor to the point of dependence increased their alcohol intake and persisted alcohol consumption despite the addition of quinine to the alcohol solution, compared with control rats that were not exposed to alcohol vapor. Acute alcohol withdrawal was accompanied by downregulated glucocorticoid receptor mRNA in various stress/reward-related brain regions, and long-term alcohol abstinence was accompanied by upregulated glucocorticoid mRNA in these brain regions. Chronic GR antagonism with mifepristone (RU38486) prevented the escalation of alcohol intake and compulsive responding induced by chronic, intermittent alcohol vapor exposure. Thus, the glucocorticoid system appears to be involved in the development of alcohol dependence and may represent a potential pharmacological target for the treatment of alcoholism. (Vendruscolo LF, et al., Journal of Neuroscience, 2012 May 30;32(22):7563-71)
Drinking in the age of the Great Recession
Unemployment, underemployment, and problematic employment situations were associated with harmful drinking outcomes, as were negative personal life experiences, such as home ownership problems, undesirable living conditions, inadequate health insurance, and constraints on personal relationships. While economic based stressors were linked to increased drinking in both genders, men were more likely to show patterns of alcohol abuse.
The United States has been experiencing the most severe economic crisis since the Great Depression. This article presents the Life Change Consequences of the Great Recession (LCCGR), an instrument depicting work and personal life-related stressors reflecting the enduring effects of the Great Recession. A national sample of 663 respondents completed a mail survey including this instrument and measures of drinking outcomes. Multiple regression analyses addressed the links between the LCCGR and drinking. Economy-related stressors manifested significant effects on both male and female consumptions patterns, but most LCCGR subscales were more clearly related to problematic drinking patterns in men compared with women. (Richman JA, et al., Journal of Addictive Diseases. 2012;31(2):158-72)
Early alcohol exposure disrupts visual cortex plasticity in mice
Research indicates that deficits in neuronal plasticity, the changes in neuronal structure, function, and organization that occurs during development, underlie the cognitive problems seen in fetal alcohol spectrum disorders (FASD). Scientists supported by NIAAA recently reported that, in a mouse model of prenatal alcohol exposure, treatments that activate both cAMP and cGMP, signaling molecules important in many biological processes, restored neuronal plasticity necessary for development of normal vision. The findings suggest that this approach may improve neuronal plasticity in FASD models.
These investigators pioneered the use of the ferret model of ocular dominance to assess the effects of third-trimester ethanol exposure on neuronal plasticity. Here they successfully translate this approach to a mouse model and demonstrate that in mice, as in ferrets, neural plasticity is sensitive to third-trimester ethanol and can be protected by a non-specific phosphodiesterase (PDE) inhibitor, suggesting that cAMP and/or cGMP mediate this protection. In addition, they showed inhibitors of cAMP-specific and cGMP-specific PDEs used in combination, but not alone, also conferred protection, indicating that a both cAMP- and cGMP-dependent mechanisms are required. These findings suggest strategies for preventing and possibly treating FASD. Furthermore, development of this mouse model creates the opportunity refine our mechanistic understanding of ethanol's effects on plasticity through the interrogation of knockout mice and other genetic constructs. (Lantz CL, Wang W, Medina AE. International Journal of Developmental Neuroscience, 2012;30(5):351-7)
Effectors of alcohol-induced cell killing in Drosophila
New research sheds lights on the cellular mechanisms triggered by alcohol that lead to apoptosis, or programmed cell death. Using fruitflies, a model that mimics alcohol’s effects on humans, the researchers identified several genes that are involved in ethanol-induced cell death. When flies were bred with a mutation to Drat (Death resistor ADH domain-containing Target), they were more likely to survive exposure to ethanol.
Heavy alcohol consumption provokes an array of degenerative pathologies but the signals that couple alcohol exposure to regulated forms of cell death are poorly understood. Using Drosophila as a model, we genetically establish that the severity of ethanol challenge dictates the type of death that occurs. In contrast to responses seen under acute exposure, cytotoxic responses to milder challenges required gene encoding components of the apoptosome, Dronc and Dark. We conducted a genome-wide RNAi screen to capture targets that specifically mediate ethanol-induced cell death. One effector, Drat, encodes a novel protein that contains an ADH domain but lacks essential residues in the catalytic site. In cultured cells and neurons in vivo, depletion of Drat conferred protection from alcohol-induced apoptosis. Adults mutated for Drat showed both improved survival and enhanced propensities toward sedation after alcohol challenge. Together, these findings highlight novel effectors that support regulated cell death incited by alcohol stress in vitro and in vivo. (Chen P, et al., Cell Death & Differentiation. 2012 April [Epub ahead of print])
Genetic dissection of acute ethanol responsive gene networks in prefrontal cortex: functional and mechanistic implications.
Sensitivity to alcohol and risk for alcohol dependence are influenced by genetic factors. In a recent study, researchers supported by NIAAA compared alcohol-induced changes in gene expression across 27 mouse lines to identify gene networks -- groups of genes that are functionally inter-related – that influence the sensitivity to alcohol. This novel approach identified previously unknown candidate genes and gene networks expressed in mouse brains that have a central role in determining the response to alcohol, and set the stage for future studies aimed at finding new therapeutic approaches for alcoholism.
Individual differences in initial sensitivity to ethanol are strongly related to the heritable risk of alcoholism in humans. To elucidate key molecular networks that modulate ethanol sensitivity we performed the first systems genetics analysis of ethanol-responsive gene expression in brain regions of the mesocorticolimbic reward circuit (prefrontal cortex, nucleus accumbens, and ventral midbrain) across a highly diverse family of 27 isogenic mouse strains (BXD panel) before and after treatment with ethanol. Acute ethanol altered the expression of ~2,750 genes in one or more regions and 400 transcripts were jointly modulated in all three. Ethanol-responsive gene networks were extracted with a powerful graph theoretical method that efficiently summarized ethanol's effects. These networks correlated with acute behavioral responses to ethanol and other drugs of abuse. As predicted, networks were heavily populated by genes controlling synaptic transmission and neuroplasticity. Several of the most densely interconnected network hubs, including Kcnma1 and Gsk3β, are known to influence behavioral or physiological responses to ethanol, validating our overall approach. Other major hub genes like Grm3, Pten and Nrg3 represent novel targets of ethanol effects. Networks were under strong genetic control by variants that we mapped to a small number of chromosomal loci. Using a novel combination of genetic, bioinformatic and network-based approaches, we identified high priority cis-regulatory candidate genes, including Scn1b, Gria1, Sncb and Nell2.The ethanol-responsive gene networks identified here represent a previously uncharacterized intermediate phenotype between DNA variation and ethanol sensitivity in mice. Networks involved in synaptic transmission were strongly regulated by ethanol and could contribute to behavioral plasticity seen with chronic ethanol. Our novel finding that hub genes and a small number of loci exert major influence over the ethanol response of gene networks could have important implications for future studies regarding the mechanisms and treatment of alcohol use disorders. (Wolen AR, et al., PLoS One, 2012, 7(4):e33575)
GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis.
A brain region called the bed nucleus of the stria terminalis (BNST) is known to play a critical role in alcohol withdrawal and relapse to alcohol use. A key molecular regulator in the BNST is the NMDA receptor, which binds to a brain chemical called glutamate. Using a unique combination of experimental methods, researchers supported by NIAAA discovered the central role that a specific part of the NMDA receptor plays in regulating alcohol’s actions in the brain, a finding that could suggest novel treatments for alcohol withdrawal and relapse.
The bed nucleus of the stria terminalis (BNST) is a critical brain area in integrating stress and reward processes. Previous studies have established that glutamate synaptic plasticity in the BNST plays an important role in the negative reinforcement of drug and alcohol dependence. However, the underlying mechanisms of ethanol actions remain largely unknown. In this study, Wills et al. examined the subunit specificity of NMDA receptors in mediating ethanol effects in the BNST. Combining conditional gene knock and pharmacological approaches, the authors revealed that GluN2B (NR2B) subunit of NMDA receptors played a key role for both the inhibitory action of acute ethanol exposure and the enhancement of the long-term potentiation (LTP) by chronic intermittent ethanol exposure. In addition, the facilitative effect of the chronic ethanol on the LTP was achieved through extrasynaptice GluN2B containing NMDA receptors. These results suggest the importance of both the NMDA receptor subunit specificity and the localization in mediating ethanol actions in the BNST. (Wills TA, et al., Proceedings of the National Academy of Sciences U S A, 2012 Jan 31; 109(5):E278-87)
Heritability of level of response and association with recent drinking history in nonalcohol-dependent drinkers
The findings shed light on the heritability of level of response to drinking and patterns of drinking among understudied African American social drinkers. The study suggests a strong genetic influence on level of response to alcohol based on an analysis of self-reported effects.
Level of response (LR) to alcohol has been shown to be associated with the risk of developing alcohol dependence and can be measured using the self-rating of the effects of alcohol (SRE) questionnaire. This study examined the heritability of the SRE-measured LR and the relationship between LR and recent alcohol drinking history (RDH) in a predominantly African American nonalcohol-dependent population. This was a sibling study of 101 social drinkers aged 21 to 35 years recruited from the Washington, DC metropolitan area. Participants were administered the SRE to assess LR and the timeline followback (TLFB) to assess RDH. The indices of SRE used were total SRE score (SRTT), early drinking SRE score (SRED), regular drinking SRE score (SRRD), and heavy drinking SRE score (SRHD). Pearson's product-moment correlation and linear regression were used to analyze SRE indices and RDH variables (quantity and drinks per drinking occasion). Heritability analysis was conducted using Sequential Oligogenic Linkage Analysis Routines (SOLAR) software with SRE indices as traits of interest. There was a significant relationship between SRE and RDH measures. Drinks per drinking day, maximum drinks, and quantity of drinks were significantly associated with SRTT, SRHD, and SRRD (all p < 0.05). SRTT showed significant heritability (h(2) = 0.67, p = 0.025), however, the SRE subindices (SRED, SRRD, SRHD) were not significantly heritable. Analysis performed in the subset consisting of only African Americans (n = 86) showed similar trends. LR, as measured by the SRE, is associated with RDH. The high level of heritability of the SRE total score suggests that genetics accounts for a significant proportion of the variation in the LR to alcohol in social drinkers. (Kalu N, et al., Alcoholism: Clinical and Experimental Research, 2012 Jun;36(6):1034-41)
Housing in environmental complexity following wheel running augments survival of newly generated hippocampal neurons in a rat model of binge alcohol exposure during the third trimester equivalent
Findings in an animal model suggest that integrating exercise and environmental complexity may be beneficial to children with fetal alcohol spectrum disorders (FASD). Previously, these investigators found that rat hippocampal neurons lost as a result of third-trimester-equivalent ethanol exposure could largely be replenished during adolescence with exercise. However, these newly generated neurons were found to be short-lived. In this study, they demonstrate that subsequent exposure of the animals to a stimulating environment significantly increases survival of the neurons induced by exercise.
Binge-like alcohol exposure in neonatal rats during the brain growth spurt causes deficits in adult neurogenesis in the hippocampal dentate gyrus (DG). Previous data from our laboratory demonstrated that 12 days of voluntary wheel running (WR) beginning on postnatal day (PD) 30 significantly increased the number of newly generated cells evident in the DG on PD42 in both alcohol-exposed (AE) and control rats, but 30 days later a sustained beneficial effect of WR was evident only in control rats. This study tested the hypothesis that housing rats in environmental complexity (EC) following WR would promote the survival of the newly generated cells stimulated by WR, particularly in AE rats. On PD4 to 9, pups were intubated with alcohol in a binge-like manner (5.25 g/kg/d), sham-intubated (SI), or reared normally. In Experiment 1, animals were either assigned to WR during PD30 to 42 or socially housed (SH). On PD42, animals were injected with bromodeoxyuridine (BrdU; 200 mg/kg) and perfused 2 hours later to confirm the WR-induced stimulation of proliferation. In Experiment 2, all animals received WR on PD30 to 42 and were injected with BrdU on the last full day of WR. On PD42, animals were randomly assigned either to EC (WR/EC) or to SH (WR/SH) for 30 days and subsequently perfused and brains were processed for immunohistochemical staining to identify BrdU+-, Ki67+-, and BrdU+/NeuN+-labeled cells in DG. In Experiment 1, WR exposure significantly increased the number of proliferating cells in all 3 postnatal conditions. In Experiment 2, the AE rats given WR/SH had significantly fewer BrdU+ cells compared with control rats given WR/SH. However, WR/EC experience significantly increased the number of surviving BrdU+ cells in both the AE and SI groups compared with WR/SH rats of the same neonatal treatment. Approximately 80% of the surviving BrdU+ cells in the DG across the conditions were colabeled with NeuN. WR followed by EC could provide a behavioral model for developing interventions in humans to ameliorate hippocampal-dependent impairments associated with fetal alcohol spectrum disorders. (Hamilton GF, Boschen KE, Goodlett CR, Greenough WT, Klintsova AY. Alcoholism: Clinical and Experimental Research, 2012;36(7):1196-204, 2012)
Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury
Studies suggest that probiotics can treat alcohol-induced liver injury associated with gut leakiness and endotoxemia, however the mechanism is poorly understood. Researchers tested the effectiveness of the probiotic Lactobacillus rhamnosus Gorbach-Goldin (LGG) pretreatment and found that it protected against acute alcohol-induced liver injury by promoting signaling of hypoxia-inducible factor (HIF), an important transcription factor for proteins that play a role in intestinal permeability. Further studies of this probiotic could potentially advance the development of new therapeutic strategies for treating alcoholic liver disease.
Endotoxemia is a contributing cofactor to alcoholic liver disease (ALD), and alcohol-induced increased intestinal permeability is one of the mechanisms of endotoxin absorption. Probiotic bacteria have been shown to promote intestinal epithelial integrity and protect barrier function in inflammatory bowel disease (IBD) and in ALD. Although it is highly possible that some common molecules secreted by probiotics contribute to this action in IBD, the effect of probiotic culture supernatant has not yet been studied in ALD. We examined the effects of Lactobacillus rhamnosus GG culture supernatant (LGG-s) on the acute alcohol-induced intestinal integrity and liver injury in a mouse model. Mice on standard chow diet were supplemented with supernatant from LGG culture (10(9) colony-forming unit/mouse) for 5 days, and one dose of alcohol at 6 g/kg body wt was administered via gavage. Intestinal permeability was measured by FITC-FD-4 ex vivo. Alcohol-induced liver injury was examined by measuring the activity of alanine aminotransferase (ALT) in plasma, and liver steatosis was evaluated by triglyceride content and Oil Red O staining of the liver sections. LGG-s pretreatment restored alcohol-induced reduction in ileum mRNA levels of claudin-1, intestine trefoil factor (ITF), P-glycoprotein (P-gp), and cathelin-related antimicrobial peptide (CRAMP), which play important roles on intestinal barrier integrity. As a result, LGG-s pretreatment significantly inhibited the alcohol-induced intestinal permeability, endotoxemia and subsequently liver injury. Interestingly, LGG-s pretreatment increased ileum mRNA expression of hypoxia-inducible factor (HIF)-2α, an important transcription factor of ITF, P-gp, and CRAMP. These results suggest that LGG-s ameliorates the acute alcohol-induced liver injury by promoting HIF signaling, leading to the suppression of alcohol-induced increased intestinal permeability and endotoxemia. The use of bacteria-free LGG culture supernatant provides a novel strategy for prevention of acute alcohol-induced liver injury. (Wang Y, et al., American Journal of Physiology – Gastrointestinal and Liver Physiology, 2012 Jul;303(1):G32-41)
Male Germline Transmits Fetal Alcohol Adverse Effect on Hypothalamic Proopiomelanocortin gene Across Generation
This is the first study to show that alcohol exposure in the womb may lead to epigenetic changes in the POMC gene, which are then passed down through the generations by male offspring. Epigenetic changes can lead to improper “activation” or “silencing” of genes and are thought to be one way in which environmental factors may cause long-term biological or behavioral effects. These changes in POMC gene expression may play a critical role in alcoholism-inherited diseases and have implications for other diseases such as obesity, metabolic disorders, and cancer.
Neurons containing proopiomelanocortin (POMC)-derived peptides, known to control stress axis, metabolic, and immune functions, have a lower function in patients with a family history of alcoholism, suggesting that alcohol’s effects on the POMC system may transmit through generations. This paper describes the epigenetic modifications of the POMC gene that transmit through generations via the male germline and may be critically involved in alcoholism-inherited diseases. The authors found that fetal alcohol exposure increases methylation of the POMC gene by histone modification and DNA methylation in the hypothalamus, that POMC gene hypermethylation results in stress axis hyper-response, and that the POMC system abnormality is transmitted across generations via the male germline. The findings of this paper bring an important perspective to the epigenetic inheritance because it is the first study to show that alcohol exposure during the developmental period incites epigenetic marks in the gene leading to permanent alteration of POMC gene expression in the hypothalamus that propagate through generations. Moreover, the findings could have important implications for research on the epigenetic control of POMC in the inheritance of other diseases including obesity, metabolic disorders, and cancer (Govorko D, Bekdash RA, Zhang C, Sarkar DK. Biological Psychiatry , 2012 May 22 [Epub ahead of print])
Overexpression of serum response factor in astrocytes improves neuronal plasticity in a model of early alcohol exposure
The findings have implications for potentially treating FASD and other neurocognitive disorders using strategies that enhance the activity of serum response factor (SRF) in astrocytes, star-shaped cells in the brain and spinal cord. SRF is a protein that controls the flow of genetic information during transcription, thereby playing a role in cell growth, cell differentiation, and cell cycle regulation. A prior study demonstrated that brain plasticity in a ferret model of FASD could be restored using forced expression of serum response factor in the visual cortex.
Neuronal plasticity deficits underlie many of the cognitive problems seen in fetal alcohol spectrum disorders (FASD). We have developed a ferret model showing that early alcohol exposure leads to a persistent disruption in ocular dominance (OD) plasticity. Recently, we showed that this deficit could be reversed by overexpression of serum response factor (SRF) in the primary visual cortex during the period of monocular deprivation (MD). Surprisingly, this restoration was observed throughout the extent of visual cortex and most of the cells transfected by the virus were positive for the astrocytic marker GFAP rather than the neuronal marker NeuN. Here we test whether overexpression of SRF exclusively in astrocytes is sufficient to restore OD plasticity in alcohol-exposed ferrets. To accomplish that, first we exposed cultured astrocytes to Sindbis viruses carrying either a constitutively active form of SRF (SRF+), a dominant negative (SRF-) or control Green Fluorescent Protein (GFP). After 24h, these astrocytes were implanted in the visual cortex of alcohol-exposed animals or saline controls one day before MD. Optical imaging of intrinsic signals showed that alcohol-exposed animals that were implanted with astrocytes expressing SRF, but not SRF- or GFP, showed robust restoration of OD plasticity in all visual cortex. These findings suggest that overexpression of SRF exclusively in astrocytes can improve neuronal plasticity in FASD (Paul AP, Medina AE. Neuroscience, 2012 [in press])
Pilot Study of iPS-Derived Neural Cells to Examine Biologic Effects of Alcohol on Human Neurons in Vitro
Studies of the molecular mechanisms underlying many of the behavioral and biologic causes and effects of alcohol problems remain constrained by a dependence on rodent or postmortem human brain models. To help overcome those constraints, researchers supported by NIAAA recently reported that the molecular effects of acute and chronic alcohol exposure can be studied in nervous system tissue derived from “reprogrammed” human skin cells grown in culture. This pilot study demonstrates that the generation of neural tissue from skin cells could become a valuable tool for alcohol researchers.
Studies of the effects of alcohol on N-methyl-D-aspartate (NMDA) receptor function and gene expression have depended on rodent or postmortem human brain models. Ideally, the effects of alcohol might better be examined in living neural tissue derived from human subjects. In this study, the authors used new technologies to reprogram human subject-specific tissue (skin biopsies) into pluripotent cell colonies and generated human neural cultures as a model system to examine the molecular actions of alcohol. Induced pluripotent stem (iPS) cells were generated from skin biopsies taken from 4 alcohol-dependent subjects and 3 social drinkers. The investigators examined changes in mRNA expression of the NMDA receptor subunit genes after 7 days of alcohol exposure and after 24-hour withdrawal from chronic alcohol exposure. Acute alcohol exposure significantly attenuated the NMDA response, an effect that was not observed after 7 days of chronic alcohol exposure. After 7 days of chronic alcohol exposure, there were significant increases in mRNA expression of NMDA receptor subunit genes in cultures derived from alcoholic subjects but not in cultures derived from nonalcoholics. These findings support the potential utility of human iPS-derived neural cultures as in vitro models to examine the molecular actions of alcohol on human neural cells. (Lieberman R, Levine ES, Kranzler HR, Abreu C, Covault J. Alcoholism: Clinical & Experimental Research, 2012 April 6 [Epub ahead of print])
Programmed Cell Death 4 (PDCD4): A Novel Player in Ethanol-Mediated Suppression of Protein Translation in Primary Cortical Neurons and Developing Cerebral Cortex
A study by researchers supported by NIAAA recently found that alcohol-induced over-expression of PDCD4, a molecule that regulates the production of proteins, is both necessary and sufficient for inhibiting protein synthesis in the developing brain. The study showed that alcohol induces PDCD4 in the embryonic cerebral cortex and other brain regions. The findings suggest that targeting PDCD4 might be a useful strategy for preventing FASD.
Prenatal exposure to ethanol (EtOH) elicits a range of neuro-developmental abnormalities, microcephaly to behavioral deficits. Impaired protein synthesis has been connected to pathogenesis of EtOH-induced brain damage and abnormal neuron development. However, mechanisms underlying these impairments of protein synthesis are not known. In this study, we illustrate the effects of EtOH on programmed cell death protein 4 (PDCD4), a tumor and translation repressor. Primary cortical neurons (PCNs) were treated with 2.5 and 4 mg/ml EtOH for different time points (4 to 24 hours), and PDCD4 expression was detected by Western blotting. Protein synthesis was determined using [35S] methionine incorporation assay. Methyl cap pull-down assay was performed to establish the effect of EtOH on association of eukaryotic initiation factor 4A (eIF4A) with capped mRNA. Luciferase assay was performed to determine the in vivo translation. A 2-day acute 5-dose binge model with EtOH (4 g/kg body wt, 25% v/v) was performed in Sprague–Dawley rats at 12-hour intervals and analyzed for PDCD4, eIF4A, and eIF4A–methyl cap association. EtOH increased PDCD4 expression in a time- and dose-dependent manner in PCNs, which inhibited the association of eIF4A with methyl cap. EtOH and ectopic PDCD4 expression suppressed in vivo translation in PCNs and RNAi targeting of PDCD4 blocked the inhibitory effect of EtOH on protein synthesis. In utero exposure of pregnant rats to EtOH resulted in a significant increase in PDCD4 in fetal cerebral cortex along with the inhibition of methyl cap–associated eIF4A, compared with isocaloric controls. Increased PDCD4 also occurred in pooled fractions of remaining brain regions. Our data, for the first time, illustrate that PDCD4 mediates inhibitory effects of EtOH on protein synthesis in PCNs and developing brain. (Narasimhan M, et al., Alcoholism: Clinical and Experimental Research, 2012 [in press])
Social Adversity, Stress and Alcohol Problems: Are Racial/Ethnic Minorities and the Poor more vulnerable?
The study suggests that racial disparities in alcoholism result from greater exposure to chronic stressors such as social adversity, rather than a greater vulnerability to such stressors. The poor, however, did appear more vulnerable to stressors, showing a stronger link between unfair treatment, depressive symptoms, and heavy drinking.
The study examined whether populations differ in their vulnerability to the effects of social adversity on psychological stress and the effects of psychological stress on alcohol problems. Data from the 2005 U.S. National Alcohol Survey (N = 4,080 adult drinkers) were analyzed using structural equation modeling to assess an overall model of pathways linking social adversity, depressive symptoms, heavy drinking, and alcohol dependence. Multiple group analyses were conducted to assess differences in the model’s relationships among Blacks versus Whites, Hispanics versus Whites, and the poor (income below the federal poverty line) versus non-poor (income above the poverty line). The overall model explained 48% of the variance in alcohol dependence and revealed significant pathways between social adversity and alcohol dependence involving depressive symptoms and heavy drinking. The effects of social adversity and depressive symptoms were no different among Blacks and Hispanics compared with Whites. However, the poor (vs. non-poor) showed stronger associations between unfair treatment and depressive symptoms and between depressive symptoms and heavy drinking. Findings suggest that racial disparities in alcohol problems may be more a function of racial/ethnic minorities’ greater exposure, rather than vulnerability, to chronic stressors such as social adversity. However, observed differences between the poor and non-poor imply that differential vulnerability contributes to socioeconomic disparities in alcohol problems. (Mulia N, Zemore SE. Journal of Studies on Alcohol and Drugs, 2012;73:570-580)
Unhealthy Alcohol and Illicit Drug Use are Associated with Decreased Quality of HIV Care
A study of veterans with HIV found that those with drug and alcohol problems often have difficulty receiving the best care. Although the overall quality of care was high for HIV-infected veteran patients in general, gaps for those with unhealthy alcohol and illicit drug use still need to be addressed. Effective implementation of behavioral and pharmacological interventions for substance use among HIV patients could greatly improve the quality of care being received.
HIV-infected patients with substance use experience suboptimal health outcomes, possibly due to variations in care. Researchers collected self-report substance use data and abstracted 9 HIV quality indicators (QIs) from medical records. Independent variables were unhealthy alcohol use (AUDIT-C score ≥4) and illicit drug use (self-report of stimulants, opioids, or injection drug use in past year). Main outcome was the percentage of QIs received, if eligible. We estimated associations between substance use and QOC using multivariable linear regression. The majority of the 3,410 patients were male (97.4%) and Black (67.0%) with a mean age of 49.1 years (SD 8.8). Overall, 25.8% reported unhealthy alcohol use, 22% illicit drug use, and participants received 81.5% (SD=18.9) of QIs. The mean percentage of QIs received was lower for those with unhealthy alcohol use vs. not (59.3% vs. 70.0%, p<.001) and those using illicit drugs vs. not (57.8% vs. 70.7%, p<.001). In multivariable models, unhealthy alcohol use (adjusted β -2.74; 95% CI -4.23, -1.25) and illicit drug use (adjusted β -3.51 95% CI -4.99, -2.02) remained inversely associated with the percentage of QIs received. Though the overall QOC for these HIV-infected Veteran patients was high, gaps persist for those with unhealthy alcohol and illicit drug use. Interventions that address substance use in HIV-infected patients may improve the QOC received. (Korthuis PT, et al.; for the Veterans Aging Cohort Study. Journal of Acquired Immune Deficiency Syndrome. 2012 Jul 19 [Epub ahead of print])

